ÓÄÊ 636/639:614.31:615.331
METHODS OF SANITARY SURVEILLANCE FOR LIVESTOCK PRODUCTION. II. ENZYME IMMUNOASSAY (EIA) OF BACITRACIN
G.P. Kononenko, A.A. Burkin
The authors developed the test-system for detection of bacitracin (BT) on basis of indirect competitive enzyme immunoassay (EIA) with use of rabbit polyclonal antibodies against a conjugate of this antibiotic with glucosoxydase and immobilized BT conjugate with gelatin. The sensitivity of BT assay is less than 1.0 ng/ml. The possibility of EIA use for detection of residual amount of BT in livestock production (milk, meat, eggs) and for the surveillance for antibiotic addition to food is discussed.
Key words: bacitracin, immunoassay, feeds, milk, meat, eggs.
Bacitracin (BT) – the polypeptide antibiotic produced by Bacillus subtilis and B. licheniformis – is the therapeutic and anabolic preparation widely used in the world for several decades (1, 2). To determine BT contents in feed, it has been proposed several methods based on high-performance liquid-chromatography (3-6) and immunoassay (7, 8), but its still an actual problem to control the completeness of its elimination from animal tissues. The development of highly sensitive physical and chemical detection methods is objectively limited by polypeptide nature of BT and, therefore, by technique difficulties at its extraction, isolation and detection. Immunochemical methods were purposefully designed for analysis of labile physiologically active proteins and enzymes, so they can be successfully applied for selective determination of peptides in native extracts.
The purpose of this study was to obtain specific immunoreagents and to develop the method for indirect competitive solid-phase enzyme-linked immunoassay (ELISA) of bacitracin (BT) in livestock products.
Technique. The chemical substances used in the experiment were: bacitracin (B 0125), glucose oxidase (GO) (EC 1.1.3.4, G 2133) (“Sigma”, USA), glutaraldehyde (“Reanal”, Hungary), organic solvents (“Fluka”, Germany), as well as domestic products - bovine serum albumin (BSA), egg albumin (EA), gelatin (Gel) and antispecific enzyme conjugate prepared as described (9) from horseradish peroxidase (EC 1.11.1.7) and donkey antiserum to rabbit immunoglobulin. ELISA was performed on high-binding polystyrene plates (# 9018, “Costar”, USA), the optical density was measured on a flatbed photometer AKI-Ts-01 (Russia). BT protein conjugates were synthesized by reaction with glutaraldehyde (10). The reaction products were dialyzed against three changes of 1000-fold quantity of sodium chloride solution (0,5%) for 2 days. The dialysates were stored at -10... -15 °C after adding an equal volume of glycerin. The synthesis of protein conjugates was performed using the BT solution in DMF the concentration 10 ng/ml.
To obtain GO-BT (50), the solution keeping 10 mg GO (0,05 umol) in 1,5 ml distilled water was added with 4 mg BT (0,4 ml aliquot of a stock solution), which corresponded to 50-fold molar excess relative to protein. To obtain BSA-BT (25), EA-BT (25) and Gel-BT (25), solutions of BSA (7 mg, 0,1 umol), EA (4 mg, 0,1 umol) and Gel (8 mg , 0,05 umol) in 1,5 ml water were added with aliquots of BT stock solution (0,4, 0,4 and 0,2 ml) corresponding to 25-fold molar excess relative to proteins. After that, all the mixtures were added with 30 ul freshly prepared aqueous solution of glutaraldehyde (2,5%), placed on a magnetic stirrer for 2 h at room temperature, then introduced 0,2 ml of an aqueous solution of sodium borohydride (concentration - 2 mg/ml), kept periodically stirring for 1 h at 4 °C and then dialyzed.
Rabbits – grey-colored males with live weight 2-3 kg – were immunized with GO-BT conjugate (50): the 1st injection subcutaneously (10-15 points of the back) contained 100 ug immunogen in complete Freund's adjuvant, the 2nd and all subsequent injections performed at monthly intervals - 100 ug immunogen in saline. 7 days after each re-injection, the blood was collected from the edge ear vein, the serum was separated, added with an equal volume of glycerin and the samples were stored at -10…-15 °Ñ. Testing of sera were determined by competitive ELISA with concentrations of reagents providing the intensity of analytical signal (absorbance) 0,8-1,2 opt. units. For testing BT conjugates, the solid-phase antigen were their solutions (0,05 or 0,15 ug/ml) in carbonate-bicarbonate buffer (0,05 M, pH 9,5). BT working solution for competitive analysis (1 ug/ml) was prepared from a stock solution in distilled water (1 mg/ml) by stepwise dilution with water or with the mixture acetonitrile:water (84:16).
For immobilization of the conjugate, 0,2 ml its solution in a carbonate-bicarbonate buffer (0,05 M, pH 9,5) were placed in plate cells and incubated at 4 °C for 16 h. Then the cells were washed 4-5 times with phosphate-buffered saline (pH 7,5) containing Tween 20 (PBST), added 0,1 ml antibody solutions, working solutions of BT or analyzed samples in the same buffer and incubated at room temperature for 1 h, then re-washed and each cell was filled with enzyme conjugate solution (0,2 ml). After 1 h incubation, the cells were washed again, added 0,2 ml substrate solution containing o-phenylenediamine (0,4 mg/ml) and H2O2 (0,005%) in citrate-phosphate buffer (0,15 M, pH 5,0). After 45 minutes, 50 ul sulfuric acid (4 M) containing Na2SO3 (0,1 M) was placed in each cell and an optical absorbance was measured at l= 492 nm.
An optimized variant of the test system was used for analysis of milk, meat, chicken and eggs purchased in Moscow supermarkets and feeds obtained from livestock farms.
Results. The commercial biochemical reagent BT (B 0125, “Sigma”, USA) has a catalogue empirical formula corresponding to bacitracin A1 (BTA1). This peptide consists of 12 amino acid residues forming a unique structure which is thought to protect BTA1 from destruction by proteases: the molecule contains thiazoline ring formed by condensation of the carboxyl group of L-isoleucine (L-Ile1) with amino- and sulfhydryl- groups of L-cysteine (L-Cys2), as well as cyclic heptapeptide fragment consisting of L- and D-amino acids (Fig. 1).
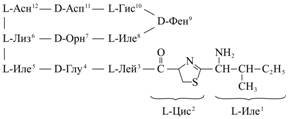 |
Fig. 1. Chemical structure of bacitracin A1. Denotations: Àñí – Asn (asparagine), Àñï – Asp (aspartic acid), Ãèñ – His (histidine), Ëèç – Lys (lysine), Îðí – Orn (ornithine), Èëå – Ile (isoleucine), Ãëó – Glu (glutamic acid), Ëåé – Leu (leucine), Öèñ – Cys (cysteine), Ôåí – Phe (phenylalanine) |
As it has been recently reported (10), chromatographic separation of the reagent allows to obtain bacitracin A1 along with
two structural analogues - bacitracin B1 and B2, whose structure contains L-valine instead of, respectively, L-Ile5 and L-Ile8 (Fig. 1), though the share of B1 and B2 products is unknown. Many authors reported about the need in a unified calibrant, in particular – the zinc bacitracin by USA pharmacopoeia (USP zinc bacitracin) (4).
The available commercial reagent BT was certified by its characteristics of the UV absorption (Table 1). Molar extinction e for a maximum absorption at wavelength 250 nm obtained by averaging the results of three measurements amounted to 3320, which value was further used to adjust the concentration of calibration solutions.
1. Characteristics of the UV absorption of bacitracin aqueous solutions |
||||
λ, nm |
C, ug/ml |
OD, opt. units |
e |
emean |
198 |
20 |
1,112 |
79 060 |
67 830 |
40 |
1,592 |
56 600 |
||
200 |
didn’t determine |
|
||
250 |
20 |
0,054 |
2950 |
3320 |
40 |
0,089 |
3160 |
||
200 |
0,415 |
3840 |
||
Note. OD – optical density, e — molar extinction, Ñ — concentration, λ — wavelength |
||||
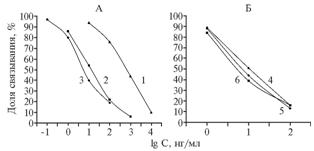 |
Fig. 2. Competitive enzyme-linked immunosorbent assay (ELISA) of bacitracin (BT) using antisera to GO-BT (50) of 1st-3rd blood samplings (1-3) and the solid-phase antigen BSA-BT (25) (A), as well as the antiserum to GO-BT (50) of the 3rd blood sapling and the solid-phase antigen Gel-BT (25) in PBST (4), in the mixture of acetonitrile with water at 10-fold dilution with PBST (5) and in milk at 3-fold dilution with PBST (6) (B). GO-BT (50), BSA-BT (25) and Gel-BT (25) – conjugates of, respectively, glucoseoxidase, bovine serum albumin and gelatin with bacitracin (BT), obtained at corresponding 50-fold and 25-fold molar excesses of BT. PBST – phosphate-buffered saline (pH 7,5) containing Tween 20 Denotations: |
The presence in BT molecule of free terminal amino group makes possible its covalent binding to any protein carrier using glutaraldehyde. Indeed, a standard method allowed to obtain the conjugates which after dialysis were used for immunization and as solid-phase antigens.
Immunization with the conjugate GO-BT (50) after the 2nd administration (at the 1st blood sampling) provided antibodies with titre 1:25 000 and possibility to determine BT at its dilution up to 10 ng/ml (Fig. 2, A). Continuation of immunization till the 3rd blood sampling was accompanied by maintaining such a high antibody titer and the raise of test sensitivity by almost two orders (Fig. 2, A).
| 2. Binding degree (%) of antibodies to GO-BT (50) in serum from the 3rd blood sampling with different immobilized antigens at presence of bacitracin | ||||
Immobilized antigen (0,05 ug/ml) |
BN, ng/ml |
|||
100 |
10 |
1 |
0,1 |
|
BSA-BT(25) |
19 |
40 |
80 |
97 |
Gel-BT(25) |
13 |
32 |
74 |
93 |
GO-BT(50) |
26 |
41 |
75 |
94 |
Note. BSA-BT (25), Gel-BT (25) and GO-BT (50),– conjugates of, respectively, bovine serum albumin, gelatin and glucoseoxidase with bacitracin (BT), obtained at corresponding 25-fold and 50-fold molar excesses of BT. |
||||
The assessment of test selectivity should consider the fact that the active ingredient of veterinarian preparations is usually BT methylene disalycilate or its complex compounds with salts of divalent metals (eg., zinc bacitracin) providing more pronounced antibacterial effects.
BT is known as capable for easy formation of complexes with divalent ions of Zn, Co, Ni and Cu in the mixed solutions with equimolar concentrations, which is accompanied by changes in its structure. The complex Co (II)-BTA1 in aqueous solution has the structure including a “tail” amino acid fragment of the molecule (L-Ile1-D-Glu4) that brings the ion Co(II) close to a single imidazole ring of L-His10 thereby linking it to cyclic geptapeptide fragment of the molecule. Since complexation of BT can lead to disorientation of individual fragments within the molecule, the authors studied how antibodies to an unbound BT recognize the complex of BT with Co(II) obtained by the method of J.D. Epperson et al. (11), which test revealed no significant differences. The equally inhibiting concentrations IC50 (50% inhibition of antibody binding) for the complex and free BT amounted to, respectively, 5,0 and 7,0 ng/ml. Apparently, complexation doesn’t significantly affect spatial configuration of the molecule, and bound forms of BT can be effectively recognized by antibodies. Interestingly, that formation of BT complexes didn’t affect spectral characteristics too - for Zn-BT, the maximum UV absorption was 252 nm at a molar extinction of 2675, which is close to that of unbound BT (12). Ligands in such complexes – a nitrogen atom in the imidazole ring of L-His10, carboxyl group of D-Glu4 and a nitrogen atom of the thiazoline ring while the N-terminal amino group is not involved in binding, which earlier allowed to perform conjugation of Zn-Bt with proteins for obtaining immunoreagents (7 ).
Further optimization of test conditions was performed only with three solid-phase antigens (Table 2), since testing the 1st serum with EA-BT (25) revealed that it didn’t provide binding to antibodies. For BT solutions with concentrations of 1-100 ng/ml, a linear analytical signal in the range 10-90% was mostly provided by Gel-BT (25), two others were inferior while being immunoreactive as well – they didn’t bind antibodies but participated in inhibition of binding (Table 2).
Using the immobilized Gel-BT (25) and BT calibration solutions under the conditions of intermediate precision performed daily or at intervals of 1-2 days (n = 10) resulted in finding the degree of antibody binding (%) with relative standard deviation less than 0,05 indicating stable operation of the test system in laboratory conditions at normal fluctuations of external factors.
Applicability of the test system for analysis of milk was assessed (Fig. 2, B). It was revealed that the 3-fold dilution of milk with PBST didn’t cause any changes in ELISA calibration curve and the lower limit of BT detection was equal to 3 ug/kg. For six samples of milk powder diluted with water prior to analysis according to instructions on their packages, analytical signal amounted to 95-101% of control. In 19 samples of pasteurized and sterilized cow milk, BT was detected in two samples at contents of, respectively 7 and 10 ug/kg, the others were “clear” . Thus, sanitary surveillance must consider the probability of BT presence in milk and using ELISA for its detection.
According to previous studies of the authors, extraction of mycotoxins from feed with the mixture acetonitrile:water (volume ratio 86:14) at the ratio of sample weight (g) to extractant volume (ml) 1:5 with subsequent 10-fold dilution of extracts with buffer caused no background effects for ELISA. Adding to PBST of 10% this extractive mixture didn’t cause much changes in analytical performance of the test-system: the degree of antibodies binding to BT solutions with concentrations of 1, 10 and 100 ng/ml equaled to 88±4, 44±2 and 13±1 % for n = 5 (Fig. 2, B). The abovementioned technique was found to be suitable for preparing feed samples, which was proved by results of testing the random assess of 80 samples. In flour meal of animal origin (12 samples), protein-vitamin-mineral supplements (11 samples) and in mixed feeds (52 of 57 samples), the binding of antibodies was 92-114% relative to control with mean values of, respectively 97, 98 and 106% . Five samples of mixed feeds were found to be test-positive, but the established contents of BT (0,05, 0,05, 0,20, 1,20 and 2,90 mg/kg) were several orders below than therapeutic and anabolic doses. Probably, these were the cases of feeding small doses of BT as preventive supplement (13). In general, it can be assumed that highly sensitive ELISA (0,05 mg/kg) has no prospects for practical use in assessment the normality of BT introduction in feeds, although there have been some studies of this issue (7, 8).
For other livestock products (meat, eggs), extraction of antibiotics with organic solvents can be also appropriate at prior dehydration of samples (lyophilization, heat-drying). In the authors’ experiments, the ratio 1:1 wet weight of a sample (g) to volume of extractant – the mixture of acetonitrile with water (ml) – was found to be sufficient for extraction of air-dry samples of homogenized muscle tissue and eggs. A 10-fold dilution of these extracts with buffer prior to test, as before (14), didn’t distort the results. For all three concentrations of calibrant, levels of antibody binding were most similar to the values obtained in water-acetonitrile mixture diluted with PBST (Fig. 2, B). For chicken meat extracts (n = 5), these values equaled to 87±3, 49±3 and 16±2%, for extracts of chicken eggs - 88±2, 44±2 and 14±1%. Under these conditions, the expected lower limit of detection the residual BT in meat and eggs was 10 ug/kg. According to domestic sanitary regulations, BT presence in meat and eggs is not permitted, and test method should provide reliable detection of BT content 20 ug/kg (15). In saline extracts of meat and eggs, BT can be determined at greater sensitivity even at multiple dilution.
Thus, the developed ELISA test system provides high sensitivity of determining bacitracin residues. Optimized conditions for samples preparation ensure simple and reliable detection of BT during a sanitary surveillance of all types of livestock products.
REFERENCES
1. Klenova I.F. and Yaremenko N.A., Veterinarnye preparaty v Rossii: Spravochnik (Veterinary Preparations in Russia: Reference Book), Moscow, 2001.
2. Swick R.A., Role of Growth Promotants in Poultry and Swine Feed, Technical Bulletin ASA, 1996, vol. AN04, pp. 1-10.
3. Ragheb H.S., Evaluation of Methanol Extraction for Determination of Bacitracin in Feeds, J. Assoc. Anal. Chem., 1980, vol. 63, no. 3, pp. 444-447.
4. Ragheb H.S., Bacitracin Determination in Feeds: Evaluation of Methods, J. Assoc. Anal. Chem., 1981, vol. 64, no. 4, pp. 980-990.
5. Gallagher J.B., Love P.W. and Knotts L.L., High Pressure Liquid Chromatographic Determination of Bacitracin in Premix Feeds and Finished Feeds: Collaborative Study, J. Assoc. Anal. Chem., 1982, vol. 65, no. 5, pp. 1178-1185.
6. Capitan-Vallvey L.F., Titos A., Checa R. and Navas N., High Pressure Liquid Chromatographic Determination of Zn-Bacitracin in Animal Feed by Post-Column Derivatization and Fluorescence Detection, J. Chromatog. A, 2002, vol. 943, no. 2, pp. 227-234.
7. Williams C., Patel I., Willer C.J. and Crosby N.T., Competitive Enzyme-Linked Immunosorbent Assay for the Determination of Zinc Bacitracin in Animal Feedingstuffs, Analyst, 1994, vol. 119, no. 3, pp. 427-430.
8. Situ C. and Elliott C.T., Simultaneous and Rapid Detection of Five Banned Antibiotic Growth Promoters by Immunoassay, Analytica Chimica Acta, 2005, vol. 529, no. 1-2, pp. 89-96.
9. Nakane P.K. and Kawaoi A., Peroxidase-Labeled Antibody. A New Method of Conjugation, J. Histochem. Cytochem., 1974, vol. 22, no. 2, pp. 1084-1091.
10. Hermanson G.T., Bioconjugate Techniques, San Diego – New York – Boston – London – Sydney – Tokyo – Toronto: Academic Press, 1996, pp. 470-472.
11. Epperson J.D. and Ming L.-J., Proton NMR Studies of Co(II) Complexes of the Peptide Antibiotic Bacitracin and Analogues: Insight into Structure-Activity Relationship, Biochemistry, 2000, vol. 39, pp. 4037-4045.
12. Clark E.G.C., Isolation and Identification of Drugs, London, 1986.
13. Leonov N.I., Skryabin G.K. and Solntsev K.M., Antibiotiki v zhivotnovodstve (Antibiotics in Animal Husbandry), Moscow, 1962.
14. Burkin A.A., Kononenko G.P. and Burkin M.A., Methods of Sanitary Control of Livestock Products. I. Immunoassay (ELISA) of Tetracyclines, S.-kh. biol., 2010, no. 4, pp. 110-117.
15. SanPiN 2.3.2.1078-01 Gigienicheskie trebovaniya k bezopasnosti i pischevoi tsennosti pischevykh productov (Sanitary and Epidemic Rules and Regulations. Food Raw Material and Foodstuff. Hygienic Requirements for Safety and Nutrition Value of Foodstuff), Moscow, 2002.
All-Russia Research and Development Institute for Veterinary Sanitation, Hygiene and Ecology, RAAS, Moscow 123022, Russia , |
Received July 1, 2010
|













