ÓÄÊ 591.05:577.161.3
GLUCOSE FERMENTATION BY Saccharomyces cerevisiae AFTER ADDITION TO MEDIUM OF EXOGENOUS NAD+ AND α-TOCOPHERYLQUINONE
V.I. Dudin
The author studied the effect of α-tocopherylquinone on glucose fermentation by dry baker's yeast Saccharomyces cerevisiae at the addition of NAD+ or NADH to incubation medium. The addition of NAD+ results in the reduction of speed of glucose involvement into glycolysis in the first 7 hours of incubation, and also the essential increase of acetaldehyde concentration in the medium. Under the action of α-tocopherylquinone the glycolysis is accelerating, and the content of acetaldehyde reduces, simultaneously. The addition of NADH to the medium raises the speed of pyruvate use.
Key words: dried baker's yeast Saccharomyces cerevisiae, glucose, acetic aldehyde, α-tocopherolquinone, pyruvic acid and acetic aldehyde formation, fermentation.
α-tocopheryl qinone is the product of free radical oxidation of vitamin E providing its main anti-oxidant effects. It is believed to be the terminal metabolite of vitamin E (1) or its active metabolite (2). It has been reported about α-tocopheryl qinone operating as an electron donor in the second stage of bio-hydrogenation of linoleic acid performed by rumen microflora (3, 4).
Most of microorganisms including yeasts have low internal osmotic pressure of 3 – 6 atm. (5) which determines high permeability of their cell membranes, osmotic type of nutrition and intense metabolic processes. In earlier experiments with Saccharomyces cerevisiae (incubation of freshly prepared or long-stored commercial dry baker's yeast in a simplified medium with D-glucose, or sodium pyruvate), the author have found that D,L-α-tocopheryl qinone accelerates fermentation during the second phase of glycolysis (6). In the variant with dry freshly prepared yeast, hexoses content in medium was almost identical in both experiment and control. Results obtained by incubation of long-term stored yeast indicate a smaller involvement of glucose in fermentation influenced by a-tocopheryl quinone during 3,5; 7 and 14 h. The decrease in acetaldehyde content could occur owing to the raise of activity of alcohol dehydrogenase caused by α-tocopheryl qinone added to the medium.
The purpose of this work was studying the effects of a-tocopheryl qinone on glucose fermentation at presence of NAD+ or NADH in incubation medium.
Technique. The object of study - commercial dry baker's yeast Saccharomyces cerevisiae (“Lesaffre”, France) the strain S11M (FXV), a substrate – D-glucose.
The 1st experiment was performed in three variants: without bioactive additives (1st control), with NAD+ added into a medium (“Sigma-Aldrich”, Germany) (2nd control) and with the addition of both NAD+ and D,L-a-tocopheryl quinone (experiment). D,L-α-tocopheryl quinone was prepared by oxidation of D,L-α-tocopherol with nitric acid (7) and the consequent purification on a silica gel column under the control of high-performance liquid chromatography (HPLC) (1). 6 g glucose was dissolved in 300 ml water and then 100 ml of this solution was poured in three flasks of 200 ml volume. In the experimental variant, 0,6 mmol NAD+ (concentration 5,48 umol/cm3) and 0,0227 mmol D,L-a-tocopheryl quinone (0,2 umol/cm3) solubilized in 10 cm3 water with 150 mg Tween-20 (“Merck”, Germany) were added into the flask. In both control variants, the same quantities of Tween-20 and water were added, and then 0,6 mmol NAD+ - into the 2nd control. After that, 0,75 g yeast were placed into each flask. The experiment was performed in 3 replicates using yeast samples from different packages. Two other variants were investigated – with NAD+ added at quantities of 0,45 mmol (4,11 umol/cm3) and 0,15 mmol (1,37 umol/cm3). Since the absence of significant differences when using yeast from different packages, these tests were carried out without replicates.
The mixtures were incubated in a water bath with shaker at 30 °C; initial samples were taken in 12 min, then – basic samples (5 ml) after 3,5, 7, 14, 24, 36, 48, 58 and 84 h. Precipitation of protein was performed using 1,25 ml 10% solution HPO3. The samples were centrifuged in the refrigerated centrifuge K 24 (“Janetzki”, Germany) at 10,000 g and +4 °C.
II experiment: 0,56 mmol (5,13 umol/cm3) NADH (“Applichem”, Germany) was added to a medium.
III experiment: a substrate for yeast - 5% solution of ethanol in water instead of glucose. Variants - the same as in the 1st experiment.
|
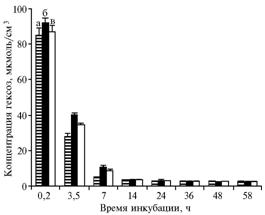
| Fig. 1. The concentration of hexoses in a medium with glucose during the incubation of yeast Saccharomyces cerevisiae: a – without additives (the 1st control), b – with 5,48 umol/cm3 NAD+ added to a medium (the 2nd control), c – with 5,48 umol/cm3 NAD+ and 0,2 umol/cm3 α-tocopheryl quinone added to a medium.
Denotation: à á â – a b c |
The content of pyruvic acid was established upon its ability to form 2,4-dinitrophenylhydrazones, which were purified by reverse extraction from toluene solution into an aqueous solution of soda (Na2CO3) and transferred to the aci-form by adding sodium hydroxide solution (8). Concentration of hexoses was determined by the method based on their dehydration leading to formation of formaldehyde, whose content was measured by reaction with chromotropic acid (9). Acetaldehyde was isolated as 2,4-dinitrophenylhydrazone from the toluene solution remaining after the extraction from it of acidic 2,4-dinitrophenylhedrazones of pyruvic acid. Toluene was evaporated and 2,4-dinitrophenylhydrazones of acetaldehyde were isolated by double chromatography with a thin bound layer of silica gel L (“Lachema”, Czech Republic) and benzene as a carrier. Hydrazones were extracted from silica gel with methanol. The content of aldehyde was determined on the spectral colorimeter Specol-11 (“Carl Zeiss”, Germany) at l = 340 nm.
Statistical processing of data was performed according to common principles of variation statistics (10) in the program Microsoft Excel.
Results. Adding to the incubation medium of 5,48 umol/cm3 NAD+ reduced the rate of glucose involvement in glycolysis (Fig. 1). Thus, for the 2nd and 3rd variants of I experiment, the significance of differences relative to the 1st control at 3,5-hour incubation (40,38 ± 0,80 vs. 28,35 ± 0,70 and 34,82 ± 1,14 vs. 28,35 ± 0,70 umol/cm3) amounted to P <0,0003 and P <0,008, respectively. α-tocopheryl quinone enhanced utilization of glucose during the 3,5-hour incubation (34,82 ± 1,14 vs. 40,38 ± 0,80 umol/cm3, P <0,02). After 7 h, differences between all variants were leveled and concentration of hexoses remained within 2-3,5 umol/cm3 until the end of experiment.
|
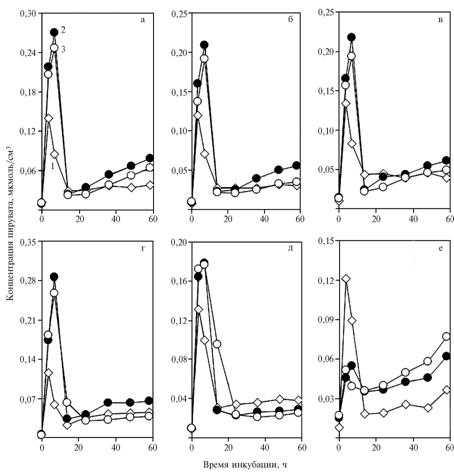
| Fig. 2. The concentration of pyruvate in a medium with glucose during the incubation of yeast Saccharomyces cerevisiae: 1 – without additives; 2 – with NAD+ added to a medium at quantities of 5,48 (a-c), 4,11 (d) and 1,37 umol/cm3 (e) or NADH at the quantity of 5,13 umol/cm3; 3 – with NAD+ (a-e) or NADH (f) and 0,2 umol/cm3 a-tocopheryl quinone added to a medium.
Denotation: à á â ã ä å – a b c d e f |
A similar dynamics was observed for pyruvate content in the medium with 5,48 or 4,11 umol/cm3 NAD+ (Fig. 2, a-d). The peak of pyruvate content shifted relative to control from 3,5 h to 7 h of incubation. The addition of 1,37 umol/sm3 NAD+ contributed to a less pronounced shift of the peak. These changes could be the result of raising need in reducing equivalents connected with exogenous supply of the pool of oxidized NAD. α-tocopheryl quinone inhibited formation of pyruvate, which was reflected by decreasing concentration of acetaldehyde detected between 7 and 14 h of incubation.
In II experiment, the addition of 5,13 umol/cm3 NADH to a medium sharply increased utilization of pyruvate (Fig. 2, f).
|
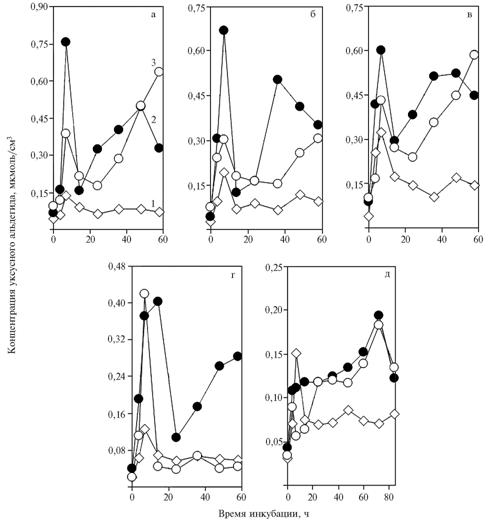
| Fig. 3. The concentration of acetaldehyde in a medium with glucose during the incubation of yeast Saccharomyces cerevisiae: 1 – without additives; 2 – with NAD+ added to a medium at quantities of 5,48 (a-c), 4,11 (d) and 1,37 umol/cm3 (e); 3 – with NAD+ and α-tocopheryl quinone added to a medium.
Denotation: à á â ã ä – a b c d e |
After the addition of 5,48 umol/cm3 NAD+, α-tocopheryl quinone and 7-h incubation, the concentration of acetaldehyde significantly reduced (0,372 ± 0,038 vs. 0,678 ± 0,043 umol/cm3 in the 2nd control; P <0,007) (Fig. 2, a-c). Similar differences (0,768 ± 0,050 vs. 1,129 ± 0,046 umol/cm3 in the 2nd control; P <0,007) were observed for the sum of three periods (3,5 + 7 + 14 h). Therefore, acceleration of glycolysis by a-tocopheryl quinoneis accompanied with increased utilization of acetaldehyde at the end of reaction sequence or, possibly, it occurs owing to this process.
These changes could be provided by direct effect of α-tocopheryl quinone(as an allosteric effector) on alcohol dehydrogenase (ADH). Reducing the dose of NAD+ to 4,11 umol/cm3 didn’t affect general regularities (Fig. 3 a-d), to 1,37 umol/cm3 – contributed to elimination of the peak in the beginning of incubation and deep minimum on the curve appeared after adding α-tocopheryl quinone(Fig. 3, e). The second maximum on the chart was probably connected with slowing of decarboxylation of pyruvate (Fig. 2, e).
Yeast alcohol dehydrogenase (alcohol: NAD+-oxidoreductase, EC 1.1.1.1) is the tetramer molecule of 150 kDa weight containing 8 zinc ions. The active site of each subunit includes 2 zinc ions, one of which provides catalytic function, the second - maintaining conformation of ADH. Deletion of zinc ions from the enzyme violates its ability to cooperative unfolding (11). The presence of NAD+ strengthens the bond between monomers, but NADH reduces the strength of the structure (12). Binding of NAD+ to liver alcohol dehydrogenase leads to elimination of proton from the system of hydrogen bonds formed by Zn2+, a water molecule, serine-48 and histidine-51.
|
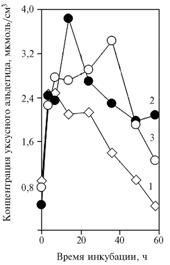
| Fig. 4. The concentration of acetaldehyde in a medium with glucose during the incubation of yeast Saccharomyces cerevisiae: 1 – without additives; 2 – with NAD+ added to a medium (2,74 umol/cm3); 3 – with NAD+ and α-tocopheryl quinone added to a medium.
Denotation: abscissa – Time of incubation, h ordinate – Concentration of acetaldehyde, umol/cm3 |
In yeast cells, there are three ADH isoenzymes containing equal amounts of zinc (13). ADH I participates in formation of ethanol during fermentation and is not subject to catabolite repression. ADH II (cytosol) and ADH III (mitochondria) are involved in oxidative metabolism of ethanol during fermentation. When glucose is present in a medium, these isoenzymes are not being synthesized (14).
The structure of lactate dehydrogenase, malate dehydrogenase, alcohol dehydrogenase and glyceroaldehyde-3-phosphate dehydrogenase contains NAD+-binding domain – the protein fragment consisting of six parallel b-chains and several a-spiral segments which binds to the coenzyme (15). The yeast ADH carries four NAD+-binding domains.
The repeated increase in acetaldehyde content after adding of NAD+ to the medium could be explained by raising utilization of ethanol (Fig. 3), most likely due to ADH II. The introduction of α-tocopheryl quinonemodifies this effect, because the second peak of acetaldehyde content appears later than the incubation period reflected in the chart. Estimation of the area occupied by this peak suggests that it could be significantly higher than the control one. In this regard, smaller height of the first concentration peak compared with control reflects increased utilization of pyruvate affected by α-tocopheryl quinone.
In III experiment, the variants with adding 2,74 umol/cm3 NAD+ revealed the increased formation of acetaldehyde relative to control (Fig. 4). The introduction of a-tocopheryl quinoneresulted in shift of acetaldehyde content peak to later time of incubation. The prolonged incubation could result in greater amount of ethanol formed and oxidized by the added NAD+.
Thus, the effects of α-tocopheryl quinone on fermentation are provided by co-enzyme function of NAD+, but not NADH. Introduction into a medium of NAD+ reduced the rate of hexoses involvement in sequence of glycolytic reactions during the first 7 h of incubation and provided significant raise of acetaldehyde content in a medium. α-tocopheryl quinoneaccelerated glycolysis, therefore, the content of acetaldehyde decreased. NADH added into a medium enhanced the rate of pyruvate utilization.
REFERENCES
1. Dudin B.I., Biokhimiya vitamina E i svyazannykh s nim biologicheski aktivnykh veschestv (Biochemistry of Vitamin E and Related Substances), Moscow, 2004.
2. Donchenko G.V., Biokhimiya ubikhinona (Biochemistry of Ubiquinone), Kyiv, 1988.
3. Hughes P.E. and Tove S.B., Identification of an Endogenous Electron Donor for Biohydrogenation as a-Tocopherolquinol, J. Biol. Chem., 1980, vol. 255, no. 10, pp. 4447-4452.
4. Hughes P.E., Hunter W.J. and Tove S.B., Biohydrogenation of Unsaturated Fatty Acids, J. Biol. Chem., 1982, vol. 257, no. 7, pp. 3643-3649.
5. Konovalov S.A., Biokhimiya drozhzhei (Biochemistry of Yeast), Moscow, 1962.
6. Dudin V.I., About Participation of a-Tocopherilquinone in Glucose Fermentation by Saccharomyces cerevisiae, S.-kh. biol., 2010, no. 2, pp. 34-39.
7. Rao G.H.R., Keich T.P. and White J.G., Preparation, Separation and Characterisation of Vitamin E Quinone, J. Chromat., 1980, vol. 196, no. 3, pp. 506-511.
8. Petrun’kina A.M., Prakticheskaya biokhimiya (Practical Biochemistry), Leningrad, 1961, pp. 384-390.
9. Korenman I.M., Metody opredeleniya organicheskikh soedinenii (Methods for the Determination of Organic Compounds), Moscow, 1970, pp. 65-66.
10. Merkur’eva E.Kl, Biometriya v zhivotnovodstve (Biometry in Animal Husbandry), Moscow, 1964.
11. Yang I. and Zhou Kh.-M., Effect of Zinc Ions of Conformational Stability of Yeast Alcohol Dehydrogenase, Biokhimiya, 2001, vol. 66, no. 1, pp. 61-70.
12. White A., Handler Ph., Smith E., Hill R. and Leman I., Osnovy biokhimii (Basics of Biochemistry), Vol. I, Moscow, 1981.
13. Eichhgorn G., Neorganicheskaya biokhimiya (Inorganic Biochemistry), Moscow, 1978.
14. Berry D., Biologiya drozhzhei (Biochemistry of Yeast), Moscow, 1985.
15. Anisimov A.A., Osnovy biokhimii (Basics of Biochemistry), Moscow, 1986.
ÃÍÓ Âñåðîññèéñêèé ÍÈÈ ôèçèîëîãèè, |
Ïîñòóïèëà â ðåäàêöèþ |