doi: 10.15389/agrobiology.2012.4.73eng
УДК 636.52/.58:619:578.891:578.5
HEPATITIS SPLENOMEGALY SYNDROME, A NEWLY IDENTIFIED HEN VIRAL DISEASE IN RUSSIA
V.N. Irza, A.V. Sprygin
For the first time in Russia, the viral agent of hepatitis splenomegaly syndrome, a disease of laying hens from broilers’ parental flock, has been identified by the use of RT-PCR (reverse transcription — polymerase chain reaction). On a poultry farm a low laying ability and high death of sick birds were examined, and the dissection revealed the bloody liquid in abdominal cavity, the hepato- and splenomegaly, the hepatic subcapsular hematomas and a breakable consistence of the liver. The viruses that cause similar clinical symptoms (adenovirus, Marek’s disease virus, leucosis virus) were not found. The sequence analysis of DNA fragment of ORF2 capsid protein gene of isolated virus and subsequent comparison with analogous sequences from GenBank database (NCBI) confirmed the identity of the agent to hepatitis E virus. Amplicon sequencing showed 61.6-78.6 % homology to the sequences from GenBank for capsid protein ORF2 gene of hepatitis E virus.
Keywords: avian hepatitis E virus, hens, hepato- and splenomegaly, genetic identification.
Avian hepatitis E virus, or aHEV, is a relatively novel disease of chickens with dual manifestation: big liver and spleen disease, BLS (1) or hepatitis-splenomegaly, HSS (2). These symptoms occur against the decline in egg production and increased mortality. aHEV is histologically expressed with nonspecific hepatitis, though HSS - with fibrinoid necrosis, infiltration of lymphocytes, periphlebitis, and accumulation of homogeneous eosinophilic protein in the liver sinuses.
The pathogen of avian hepatitis E belongs to the family Hepeviridae; it is icosahedral, non-enveloped, containing a single-stranded RNA virus with positive polarity (3) Currently, there are four identified genotypes of hepatitis E, 3rd and 4th of which affect livestock (respectively, pigs and chickens). However, the 4th genotype is tentatively attributed to avian hepatitis E virus, which has an intermediate position within the family (8). Possibly, in the future aHEV will be given the status of genus.
Viral hepatitis of chickens was diagnosed in Australia (4), United States (5, 6), Spain (7), Hungary (9), Italy (10) and China (11). In Russia, it hasn’t been yet identified by techniques of molecular biology, but certain reports indicate aHEV circulation in poultry farms of Russia, and, moreover, aHEV seropositivity was confirmed in nearly 18,3% chickens (12).
The purpose of this study was genetic identification of chicken hepatitis E virus agent in Russia.
Technique. The object of study were laying hens bred in a broiler farm in the territory of Russia; reduced egg production and increased mortality were observed in parental flock of this farm. Postmortem studies of hens with hepatomegaly and splenomegaly symptoms, as well as PCR of their biological material (samples of the liver tissue and bile) were conducted in 2009-2010.
Total RNA was prepared from the homogenate of liver and bile using a kit for nucleic acid extraction with sorbent NucleoS+ (“Biokom”, Moscow) according to manufacturer's instructions. Polymerase chain reaction with reverse transcription (RT-PCR) was performed in a prescribed protocol on the device “Tertsik” (“DNA technology”, Moscow) using primers for genes of aHEV helicase and capsid protein (13). The reactions were done using reagents and Taq-polymerase by “Promega” (USA). PCR products were separated in 1% agarose gel with ethidium bromide; the result was considered positive if the detection of expected amplicon length (13). All positive samples were sequenced with the use of appropriate primers on an automatic sequencer ABI Prism 3130 (“Applied Biosystems”, USA).
The obtained sequences were compared with reference sequences from GenBank of NCBI (National Center for Biotechnology Information). Sequence alignment was performed in Clustal W program, dendrograms were designed through the algorithm of “neighbor-joining” with a bootstrap parameter of 1000 repeats (MEGA 3.1).
Results. In 2009-2010, the unusual disease of laying hens was observed in parental flock of broilers - the syndrome of large liver and spleen. Veterinarians of poultry farms collected the samples of pathological material for analysis in order to exclude similarly manifested diseases – Marek's disease, leukemia and adenoviral infections. A postmortem examination of sick chickens with hepatomegaly and splenomegaly revealed in their liver clinical symptoms of viral hepatitis E (Fig. 1, see the third page of the cover).
Testing the samples for other disease agents (viruses of Marek's disease, leukemia, agents of adenovirus infections) showed a negative result (data not presented). Identification of aHEV in chickens was performed by nest-RT-PCR with two pairs of primers (12), however, a positive result was detected with only primer for capsid protein gene ORF 2 (Fig. 2). The absence of positive response to aHEV helicase gene can be explained by high genetic variability of the obtained isolate, which hypothesis though needs an additional genetic analysis.
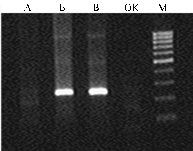 |
Fig. 2. Electrophoregram of RT-PCR products in the test for avian hepatitis E virus (aHEV) . The tested pathological material (liver tissue and bile) was derived from laying hens with hepatomegaly and splenomegaly bred in parental flock of a broiler farm in Russia: А — the absence of amplification products of aHEV helicase gene; Б В – positive result of amplification of aHEV protein ORF2 gene (fragment 242 bp length); ОК – negative control; М – molecular weight marker 100 bp DNA Ladder (“Fermentas”, Lithuania). |
|
|
Positive results of amplification were confirmed by sequencing with forward and reverse primers for ORF2 gene. The dendrogram was designed upon the analysis of nucleotide sequence of ORF2 gene fragment for capsid protein (Fig. 3). An interesting fact was that the derived aHEV isolate aHEV16050 wasn’t included in any of the genetic groups shown on dendrogram. Genetic homology of aHEV16050 in respect to ORF2 gene sequences available in GenBank was 61,6-78,6%.
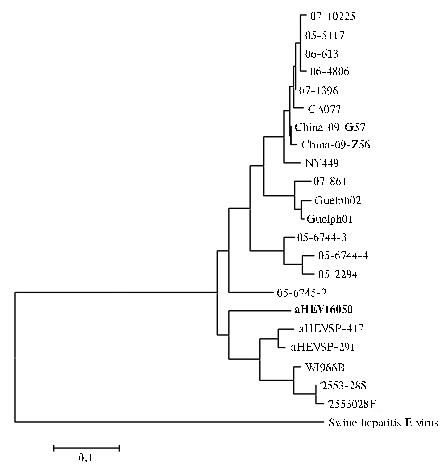 |
Fig. 3. Dendrogram of phylogenetic relations between aHEV isolate derived from laying hens with hepatomegaly and splenomegaly bred in parental flock of a broiler farm in Russia (aHEV16050) and other known isolates. The dendrogram was designed through “neighbor-joining” algorithm upon the data about nucleotide sequences of aHEV gene fragment ORF2 for capsid protein. The outgroup for comparison – analogous sequence of swine hepatitis E virus. |
The derived sequence of aHEV gene ORF2 identified in laying hens bred in broiler farm of Russia has been included in the database of GenBank with assigned number JQ814691.
It is important to note that aHEV can be detected in mainly by RT-PCR, because virus replication in a culture of chicken embryonic cells presents significant difficulties (14, 15).
Thus, this study was the first evidence of genetic identification of avian hepatitis E virus in chickens of Russia. The authors’ findings can help practicing veterinarians to perform correct diagnostics of specific clinical manifestations of this disease in a livestock of poultry farms.
REFERENCES
1. Payne C.J., Big Liver and Spleen Disease, in Diseases of Poultry, Saif Y.M., Barnes H.J., Glisson J.R., Fadly A.M., McDougald L.R., and Swayne D.E., Eds., Ames: Iowa State Press, 2005, pp. 1184-1186.
2. Shivaprasad H.L., Hepatitis Splenomegaly Syndrome, in Diseases of Poultry, Saif Y.M., Barnes H.J., Glisson J.R., Fadly A.M., McDougald L.R., and Swayne D.E., Eds., Ames: Iowa State Press, 2003, pp. 1186-1188. (on CD-ROM not in book).
3. Emerson S.U., Anderson D., Arankalle A. et al., Genus Hepevirus, in Virus Taxonomy: Eighth Report of the International Committee on Taxonomy of Viruses, Fauquet C.M., Mayo M.A., Maniloff J., Desselberger U., and Ball L.A., Eds., London: Elsevier Academic Press, United Kingdom, 2004, pp. 853-857.
4. Payne C.J., Ellis T.M., Plant S.L., Gregory A.R., and Wilcox G.E., Sequence Data Suggests Big Liver and Spleen Disease Virus (BLSV) is Genetically Rrelated to Hepatitis E Virus, Vet. Microbiol., 1999, vol. 68, pp. 119-125.
5. Haqshenas G., Shivaprasad H.L., Woolcock P.R., Read D.H., and Meng X.J., Genetic Identification and Characterization of a Novel Virus Related to Human Hepatitis E Virus from Chickens with Hepatitis Splenomegaly Syndrome in the United States, J. Gen. Virol., 2001, vol. 82, pp. 2449-2462.
6. Haqshenas G., Huang F.F., Fenaux M., Guenette D.K., Pierson F.W., Larsen C.T., Shivaprasad H.L., Toth T.E., and Meng X.J., The Putative Capsid Protein of the Newly Identified Avian Hepatitis E Virus Shares Antigenic Epitopes with That of Swine and Human Hepatitis E Viruses and Chicken Big Liver and Spleen Disease Virus, J. Gen. Virol., 2002, vol. 83, pp. 2201-2209.
7. Peralta B., Biarnes M., Ordonez G., Porta R., Martin M., Mateu E., Pina S., and Meng X.J., Evidence of Widespread Infection of Avian Hepatitis E Virus (Avian HEV) in Chickens from Spain, Vet. Microbiol., 2009, vol. 137, pp. 31-36.
8. Meng X.J., Anderson D.A., Arankalle V.A., Emerson S.U., Harrison T.J., Jameel S., and Okamoto H., Hepeviridae, in Virus Taxonomy, 9th Report of the ICTV, King A.M.Q., Adams M.J., Carstens E.B., and Lefkowitz E.J., Eds., London: Elsevier Academic Press, 2011, pp. 1021-1028.
9. Morrow C.J., Samu G., Mátrai E., Klausz A., Wood A.M., Richter S., Jaskulska B., and Hess M., Avian Hepatitis E Virus Infection and Possible Associated Clinical Disease in Broiler Breeder Flocks in Hungary, Avian Pathol., 2008, vol. 37, pp. 527-535.
10. Massi P., Tosi G., Gelmetti D., Lavazza A., Lombardi G., and Torcoli G., Big Liver and Spleen Disease in Broiler Breeders in Italy, Ital. J. Anim. Sci., 2005, vol. 4, pp. 303-305.
11. Zhao Q., Zhou E.M., Dong S.W. et al., Analysis of Avian Hepatitis E Virus from Chickens, China, Emerg Infect Dis., 2010, vol. 16, no. 9, pp. 1469-1472.
12. Tokarev O.I., Pathomorphological Characterisitcs of Thymus and Spleen of Chickens with Avian Hepatitis E Virus, Extended Abstract of Cand. Sci. Dissertation, Voronezh, 2012.
13. Sun Z.F., Larsen C.T., Dunlop A., Huang F.F., Pierson F.W., Toth T.E., and Meng X.J., Genetic Identification of Avian Hepatitis E Virus (HEV) from Healthy Chicken Flocks and Characterization of the Capsid Gene of 14 Avian HEV Isolates from Chickens with Hepatitis Splenomegaly Syndrome in Different Geographical Regions of the United States, J. Gen. Virol., 2004, vol. 85, pp. 693-700.
14. Haqshenas G., Shivaprasad H.L., Woolcock P.R., Read D.H., and Meng X.J., Genetic Identification and Characterization of a Novel Virus Related to Human Hepatitis E Virus from Chickens with Hepatitis-Splenomegaly Syndrome in the United States, J. Gen. Virol., 2001, vol. 82, pp. 2449-2462.
15. Payne C.J., Plant S.L., Ellis T.M., Hillier P.W., and Hopkinson W., The Detection of the Big Liver and Spleen Agent in Infected Tissues via Intravenous Chick-Embryo Inoculation, Avian Pathol., 1993, vol. 22, pp. 245-256.
Federal Centre for Animal Health (FGBI ARRIAH), |
Received March 25, 2012 |













