doi: 10.15389/agrobiology.2012.4.31eng
УДК 636.2:636.018:591.463.1:57.086.8
THE INDEX OF DNA FRAGMENTATION IN SPERM CHROMATIN AS A CRITERIA TO PREDICT AN INDIVIDUAL FECUNDITY IN BULLS SIRES
B.S. Iolchiev1, V.A. Bagirov1, P.M. Klenovitskiy1, V.P. Kononov1, A.V. Tadzieva2
A biological value of sperm, and, in particular, the intactness of genetic material are of the main importance for normal fecundity in males. To check the destructive changes of sperm chromatin, a DNA fragmentation index (FI) was estimated in frozen and thawed sperm samples of bulls of different breeds from the collection of the Laboratory of cryobiology, All-Russian Research Institute for Animal Breeding. The AO test with acridine orange (AO) as a staining agent was used. The index value was shown to depend on several factors and vary from 0.96 to 80 %. According to their FI values, the tested bulls were divided into four groups of high, middle, moderate and minimal fragmentation level with FI > 30.01 %, from 10.01 to 30.0 %, from 5.01 to 10.0 % and < 5.0 %, respectively. The FI parameter in tested samples was described with the Poisson function.
Keywords: DNA fragmentation, DNA fragmentation index, chromatin, sperm, fertility, acridine orange test (AOT).
Infertility is one of most significant problems of modern reproductive biology with a number of serious economic, moral, ethical and social aspects. Prevention of infertility is among topical issues of both medicine and livestock husbandry, whose findings help to obtain complementary data. Male infertility is of a special attention, because 50 % cases of infertility are associated with impaired fecundity of males.
The most important characteristic of male fertility is biological quality of semen, including integrity of genetic material. Chromatin is a structural unit of the cell nucleus and a basis of chromosomes. It consists of following major components: DNA (30-40 %), histones (30-50 %) and non-histone proteins (4-33 %). Despite the fact that spermatozoa result from a specific development of somatic cells, they are significantly distinct. One of such peculiarities is the structure of chromatin in mature spermatozoa (1).
The development of sperm cells includes three main phases: pre-meiotic, meiotic and post-meiotic phase. During maturation of spermatozoa, chromatin is undergoing total restructuring when 85% histones are replaced by specific proteins protamines, and in the end there’s almost complete absence of nucleosomal structure of DNA (1). Along with it, there occurs formation of disulfide bonds between protamine molecules, resulting in high-compact, almost crystalline structure of chromatin.
Many factors affect spermatogenesis and compaction of the genetic material. DNA damage of various types can be caused by age of an individual, infectious diseases, chronic or acute inflammatory pathologies, environmental contamination (2), temperature stress (3), radiation exposure, incomplete apoptosis (4), storage conditions of cryopreserved sperm (5), technique of freezing and thawing (6), etc. During the development, male gametes gradually lose the ability to DNA reparation, so impaired spermatozoa with fragmented DNA enter the ejaculate. Fragmentation of DNA can be caused by breaking of one or both DNA strands (4, 7).
In human, sperm DNA fragmentation (SDF) can be the reason of early embryonic mortality (3). According to Y. Liu et al. (8), the integrity of chromatin in human spermatozoa is closely related with their fertilizing ability. SDF was also found to be one of the causes of idiopathic infertility. Negative effects of SDF on male fertility has been reported by other authors as well (9-14). The negative correlation between the degree of DNA fragmentation and male fecundity was described in animals too (6, 15).
At the same time, spermatozoa with DNA fragmentation can correspond to all standard requirements, and they are capable to fertilize an ovule (9, 16). However, there is the limiting threshold of DNA fragmentation above which spermatozoa are infertile. In human, this threshold amounts to 30% according to D.P. Evenson et al. (3).
The degree of DNA fragmentation can determined in semen using direct and indirect techniques. Indirect techniques reveal the current degree of chromatin fragmentation, while direct ones evaluate DNA capability to fragmentation due to various reasons.
The purpose of this study was detection of chromatin abnormalities in bull sperm and their quantitative estimation by cytochemical technique with acridine orange staining.
Technique. The object of study were semen samples of bulls-sires of different breeds (n = 61, a collection of the Laboratory of reproductive cryobiology of the Biotechnology Center of All-Russia Research and Development Institute for Livestock Breeding) cryopreserved in straws and granules.
The degree of sperm DNA fragmentation was determined in chromatin using the test with acridine orange staining (AO-test). Spermatozoa were isolated from the semen fluid by centrifugation for 5 min at 6 thousand rpm, washed in phosphate-buffered saline (PBS, pH 6,8) and prepared smears on degreased glasses. The smears were dried for 10-15 min at room temperature and fixed for 1 h at 4 °C in ethanol-acetone mixture (1:1), then stained for 7 minutes in acridine orange solution (0,19 mg/mL) at room temperature. The stained samples were examined using a fluorescence microscope LUMAM-I3 (“LOMO”, Russia) recording images on the camera Levenhuk C-35 (“Levenhuk Ltd.”, USA). DNA fragmentation index (DFI) was determined as the ratio: the number of spermatozoa with damaged DNA (cells fluorescent in the red region of spectrum) to total number of spermatozoa. Documentation, calculations and presentation of results was performed using the software package by LLC “VideoTesT” (St. Petersburg) (17).
Results. Acridine orange (AO) is considered as one of the most effective and least toxic metachromatic dyes. In AO-stained smears of bull semen there were detected UV-fluorescent cells (l excitation = 280-320 nm) in red and green regions of the spectrum (Fig. 1, see the third page of cover). These spectral regions of fluorescence are peculiar to, respectively, fragmented and intact DNA.
|
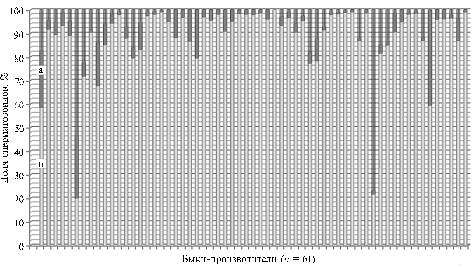
Fig. 2. The proportion of spermatozoa with fragmented DNA (a) and intact DNA (b) in semen samples of bulls-sires (acridine orange staining). |
In the studied samples of bull semen (Fig. 2) the proportion of spermatozoa with damaged chromatin averaged 11,90 ± 1,97 %. At the same time, individual integrity of the genetic material varied quite wide: the proportion of sperm with varying degrees of DNA damage ranged from 0,96 to 80 % (Fig. 2). Among the examined bulls-sires predominated individuals with low and medium index of DNA fragmentation, and only 5 animals had high DFI of 54,49±10,95 %.
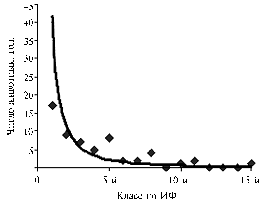 |
Fig. 3. DFI distribution of examined semen samples of bulls-sires (acridine orange staining). DFI – DNA fragmentation index; DFI grades are shown in ascending order of the proportion of spermatozoa with fragmented DNA. |
46 % animals were found to have the least damage of the genetic material in semen samples (DFI was 2,31 ± 0,21 %).
Upon these results, the studied set of semen samples of bulls-sires was considered as high-heterogeneous for DFI (Fig. 3), as well as the group of animals. In the whole group of samples, the standard deviation of DFI equaled 15,40, which indicates Poisson distribution of this trait (Fig. 3).
Depending on the degree of DNA damage in spermatozoa, the bulls-donors of semen can be divided into four groups - with high degree of DNA fragmentation in sperm cells (DFI >30,01%), medium (from 10,01 to 30,0%), moderate (5,01-10,0%), and low (<5,0%) degree of DNA fragmentation. This distribution means that results of AO-test can be useful in the development of objective criteria that help to assess the integrity of genetic material in sperm during cryopreservation, and in determination of reproductive qualities of bulls-sires.
Thus, acridine orange test has revealed in the population of spermatozoa of bulls-sires the presence of two cell types (with normal and fragmented DNA) distinct in UV-fluorescence. The degree of DNA fragmentation in the studied samples of cryopreserved bull semen was considered as quite variable - from 0,96 to 80%, which suggests the need for using the objective criteria of sperm quality at cryopreservation of semen and evaluation of reproductive function of bulls-sires.
REFERENCES
1. Bagirov V.A., Kononov V.P., Iolchiev B.A., Klenovitsky P.M., and Ernst L.K., Chromatin Status as the Index of Spermatozoa Valuable: the Tests for Estimation, S.-kh. biol., 2012, vol. 2, pp. 3-13.
2. Galimov Sh.N., Amirova Z.K., and Galimova E.F., “Human Spermatozoon Crisis” and Technogenic Contamination of Environment, Problemy reproduktsii, 2005, vol. 2, pp. 19-22.
3. Evenson D.P., Jost L.K., Corzett M., and Balhorn R., Characteristics of Human Sperm Chromatin Structure Following an Episode of Influenza and High Fever: a Case Study, J. Androl., 2000, vol. 21, pp. 739-746.
4. Agarwal A. and Said T.M., Role of Sperm Chromatin Abnormalities and DNA Damage in Male Infertility, Hum. Reprod., 2003, vol. 19, no. 4, pp. 331-345.
5. Boe-Hansen G.B., Ersboll A.K., Greve T., and Christensen P., Increasing Storage Time of Extended Boar Semen Reduces Sperm DNA Integrity, Theriogenology, 2005, vol. 63, pp. 2006-2019.
6. Bochenek M., Herjan T., Okólski A., and Smorag Z., Sperm Chromatin Abnormalities after Semen Sexing Procedure — Preliminary Results, Havemeyer Foundation Monograph Series, 2006, vol. 18, pp. 13-14.
7. Seli E. and Sakkas D., Spermatozoal Nuclear Determinants of Reproductive Outcome: Implications for ART, Hum. Reprod. Update, 2005, vol. 11, no. 4, pp. 337-349.
8. Liu Y. and Baker H.W.G., Sperm Nuclear Chromatin Normality: Relationship with Sperm Morphology, Sperm Zona Pellucida Binding, and Fertilization Rates in Vitro, Fertil. Steril., 1992, vol. 58, no. 6, pp. 1178-1184.
9. Benchaib M., Braun V., Lornage J. et al., Sperm DNA Fragmentation Decreases the Pregnancy Rate in an Assisted Reproductive Technique, Hum. Reprod., 2003, vol. 18, no. 5, pp. 1023-1028.
10. Morris I.D., Ilott S., Dixon L., and Brison D.R., The Spectrum of DNA Damage in Human Sperm Assessed by Single Cell Gel Electrophoresis (Comet Assay) and Its Relationship to Fertilization and Embryo Development, Hum. Reprod., 2002, vol. 17, pp. 990-998.
11. Tomsu M., Sharma V., and Miller D., Embryo Quality and IVF Treatment Outcomes May Correlate with Different Sperm Comet Assay Parameters, Hum. Reprod., 2002, vol. 17, no. 7, pp. 1856-1862.
12. Filatov M.V., Semenova E.V., Vorob'eva O.A. et al., Relationship between Abnormal Sperm Chromatin Packing and IVF Results, Mol. Hum. Reprod., 1999, vol. 5, pp. 825-830.
13. Host E., Lindenberg S., and Smidt-Jensen S., The Role of DNA Strand Breaks in Human Spermatozoa Used for IVF and ICSI, Acta Obstet. Gynecol. Scand., 2000, vol. 79, pp. 559-563.
14. Larson K.L., DeJonge C.J., Barnes A.M. et al., Sperm Chromatin Structure Assay Parameters as Predictors of Failed Pregnancy Following Assisted Reproductive Techniques, Hum. Reprod., 2000, vol. 15, no. 8, pp. 1717-1722.
15. Love C.C., The Sperm Chromatin Structure Assay: а Review of Clinical Applications, Anim. Reprod. Sci., 2005 Oct, vol. 89, no. 1-4, pp. 39-45.
16. Lopes S., Sun J.G., Jurisicova A. et al., Sperm Deoxyribonucleic Acid Fragmentation is Increased in Poor-Quality Semen Samples and Correlates with Failed Fertilization in Intracytoplasmic Sperm Injection, Fertil. Steril., 1998, vol. 69, pp. 528-532.
17. Iolchiev B.S., Bagirov V.A., Klenovitsky P.M., and Kononov V.P., Computer Technology for Animal Semen Evaluation, Dostizheniya nauki i tekhniki APK, 2011, vol. 9, pp. 54-56.
1Biotechnology Center of the All-Russia Research and Development Institute for Livestock Husbandry, RAAS, |
Received May 4, 2012
|