doi: 10.15389/agrobiology.2012.4.113eng
УДК 636/639:614.31
METHODS OF SANITARY SURVEILLANCE OF LIVESTOCK PRODUCTION. VI. IMMUNE-ENZYME ANALYSIS OF CHLORAMPHENICOL
M.A. Burkin1, G.P. Kononenko2, A.A. Burkin2
For monitoring of chloramphenicol residues, the conditions were optimized of an indirect immune-enzyme assay on the basis of polyclonal rabbit antibodies to chloramphenicol succinate conjugated with bovine serum albumin. Chloramphenicol succinate conjugated with a heterologous protein carrier, such as egg albumin, rabbit serum albumin and gelatin, was used as an immobilized antigen. A sensitivity of the assay makes 0.1 ng/ml. The possibility to use a developed immune-enzyme test-system in control of chloramphenicol residues in milk, meat and eggs is discussed.
Keywords: chloramphenicol, feeds, milk, meat, eggs, immunoassay.
Antibiotic chloramphenicol (CAP), or Laevomycetin, is widely used in veterinary practice of Russia for many years (1). Local researchers show the possibility of screening CAP residues in milk and meat by an indirect solid-phase competitive enzyme immunoassay (EIA) with effectively eliminated matrix effects, e.g. by introducing 1% casein in buffer solutions or dilution of a sample (2, 3). Development of highly sensitive tests of this type is a quite urgent task in the view of the minimum permissible limit of CAP in food products 0,3 mg / kg adopted in 2012 instead of the earlier one 10 mg/kg (4).
The purpose of this work was to develop a highly sensitive approach to indirect solid-phase competitive enzyme-linked immunoassay of residual chloramphenicol in major livestock products, which would meet contemporary potency requirements for such test systems.
Technique. In this experiment the authors used chloramphenicol (C-0378), chloramphenicol base (C-0135), chloramphenicol sodium succinate (C-3787), chloramphenicol glucuronide (C-9899), N-hydroxy succine imide (H-7377), water-soluble carbodiimide (E-7750), Freund's complete adjuvant (F-5881) (“Sigma”, USA), fat-free milk powder (MP; # 70166, “Fluka”, Germany), bovine serum albumin (BSA), rabbit serum albumin (RSA), egg albumin (EA), and gelatin (GEL) of domestic production. Antiserum enzyme conjugate was prepared from horseradish peroxidase (EC 1.11.1.7) and donkey antiserum to rabbit immunoglobulin according to the prescribed method (5). ELISA was performed on high-binding polystyrene plates (# 9018, “Costar”, USA) using a photometer AKI-Ts-01 (Russia). UV spectra were recorded on Hitachi-557 (“Hitachi”, Japan). Protein conjugates were synthesized through the method of activated esters following a common procedure described by G.T. Hermanson (6).
Conjugates of albumin with 25- and 50-fold excesses of CAP (BSA-CAP(25), BSA-CAP(50), RSA-CAP(25), RSA-CAP(50), EA-CAP(25), and EA-CAP(50)) were obtained by adding to 8 mg (~18 umol) CAP succinate solution in 0,5 ml dimethylformamide of 4 mg (~36 umol) N-hydroxysuccinimide and 3,5 mg (~ 18 umol) water-soluble carbodiimide, and stirred at room temperature for 1 hour. Then this mixture was added in appropriate proportion to BSA, RSA and EA (respectively, 7,0 mg; 6,5 mg, and 4 mg, or 0,1 mmol each one) solutions in 0,5 ml carbonate buffer (pH 9,6), stirred for 14 h and dialyzed.
Conjugates of GEL with 5-, 10- and 25-fold excesses of CAP (GEL-CAP(5), GEL-CAP(10), and GEL-CAP(25)) were obtained by adding to 3 mg (6,7 umol) CAP succinate of 15 mg (10 umol) N-hydroxysuccinimide and 2,9 mg (15 mmol) water-soluble carbodiimide, stirred at room temperature for 3 h. Then this mixture was added dropwise in appropriate proportion to gelatin solution (8 mg in 1,5 ml carbonate buffer, pH 9,6), stirred for 1 hour at room temperature and overnight at 4 °C, then dialyzed. The dialysis was carried out against three changes of 1000-fold volume of 0,5% sodium chloride solution for 2 days, then equal quantities of glycerol were added to the reaction product and then stored at -10...-15 °C.
Immunization of rabbits, testing antisera and ELISA procedures were performed similarly to ones described previously (7). Working solution of CAP for competitive analysis was prepared by diluting in acetonitrile of a stock solution of CAP tested spectrophotometrically (C = 20 ug/ml; l= 274 ± 1 nm; e = 9156 ± 245; n = 3). Calibration curves in the coordinates “the degree of antibody binding – the concentration of CAP solution” (n = 10) were designed at an intermediate precision daily or once in 1-2 days. Chemical identity and individuality of CAP and CAP succinate were verified by spectrophotometry and thin-layer chromatography on Silufol UV 254 plates (Czech Republic) in a mobile phase chloroform : methanol (9:1).
Tested samples: muscle tissue and internal organs (heart, liver) of chickens. CAP succinate solution (300 mg in 25 ml water) was introduced with a probe into esophagus of the first individual (test; body weight 1,44 kg) in two equal portions at intervals of 24 hours, while the second individual (control; body weight 1,50 kg) simultaneously received the same portions of water. In 5 h after the second administration, the chickens were decapitated and the samples for testing were extracted, homogenized and stored in a freezer until analysis. Other tested products: milk, meat and eggs obtained from the market network of Moscow. Preparation of samples for analysis was performed as described previously (8, 9).
The data are presented with average standard deviations.
Results. Identity and individuality of CAP and CAP succinate used in this work was confirmed by spectrometric analysis and thin-layer chromatography. Chromatographic mobility (Rf) for CAP and CAP succinate amounted to, respectively, 0,3 and 0,4. UV spectra showed a maximum UV absorption and specific absorption similar to those described (10).
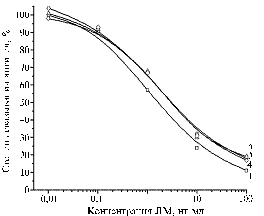
The conjugate BSA-CAP (50) used for immunization provided antibodies with working titer of 1:25 000 already after the 2nd injection (the 1st blood sample), which then increased up to 1:50 000 at the two following samplings. Later, the immune response showed a smooth dynamics (Fig. 1). Testing antisera from the 5th-7th blood samples did not reveal any difference in results.
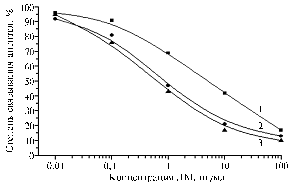 |
Fig. 1. The degree of antibody binding to BSA-CAP(50) obtained from the 2nd (1), 4th (2) and 5th-7th (3) blood sample, with solid-phase antigen GEL-CAP (5) in the presence of CAP in PBST buffer: BSA, GEL, CAP, PBST – resp., bovine serum albumin, gelatin, chloramphenicol, phosphate-buffered saline with Tween 20 (IAA). Denotations:
|
A competitive analysis with antibodies from the 3rd blood sample has shown that three immobilized conjugates – GEL-CAP(5), RSA-CAP(25) and EA-CAP(25) were superior to all other solid-phase antigens (Table 1). Being applied on a solid phase, these conjugates from solutions with a concentration of 0,05 ug/ml allowed the detection of CAP up to 0,1 ng/ml at dilution with PBST.
| 1. Degree of antibody binding (%) to BSA-CAP(50) in the serum from the 3rd blood sample with various immobilized antigens in the presence of CAP (IEA). | |||||
Immobilized antigen |
CAP, ng/ml |
||||
100 |
10 |
1 |
0,1 |
0,01 |
|
BSA-CAP(25) |
20 |
44 |
64 |
90 |
100 |
BSA-CAP(50) |
34 |
61 |
83 |
97 |
99 |
RSA-CAP(25) |
14 |
27 |
50 |
81 |
96 |
RSA-CAP(50) |
28 |
52 |
71 |
94 |
104 |
EA-CAP(25) |
16 |
36 |
60 |
84 |
98 |
EA-CAP(50) |
74 |
90 |
100 |
104 |
101 |
GEL-CAP(5) |
17 |
42 |
69 |
91 |
102 |
GEL-CAP(10) |
41 |
65 |
85 |
96 |
100 |
GEL-CAP(25) |
50 |
74 |
87 |
100 |
99 |
Note. BSA, RSA, EA, GEL, CAP – resp., bovine serum albumin, rabbit serum albumin, egg albumin, gelatin, chloramphenicol |
|||||
Right choice of an immobilized antigen is often key important for functioning of an indirect ELISA-based test system. As a rule, the best analytical performance is achieved using the immobilized antigen heterologous to immunogen and having low epitope density, such as GEL-CAP(5). The equivalent performance of BSA-CAP(25) and EA-CAP(25) are rather the exception: high sensitivity of the analysis was provided by the conjugated antigen with hapten load almost equal to that of immunogen, and albumin of a close structure used as a protein carrier. An attempt to use a conjugate heterologous in terms of the synthesis method as a solid phase antigen was performed in 1984 by USA researchers, however, the test system with antibodies to conjugate of CAP succinate with snail hemocyanin and immobilized antigen derived by mixed-anhydride reaction (protein carrier – BSA) couldn’t overcome the threshold of sensitivity of 1 ng/ml (11).
Evaluation of cross-reactivity of antibodies in respect to CAP derivatives (Table 2) showed a higher degree of recognition in CAP succinate, which was generally expected due to its use as a hapten in immunogen. It’s quite notable that the developed test system allows a close interaction with the modified CAP (glucuronide) and free CAP forms, which can jointly participate the contamination of animal biological fluids and tissues.
| 2. Cross-reactivity of antibodies to BSA-CAP(50) in the serum from the 3rd blood sample in respect to CAP derivatives and its structural analogs with immobilized antigen GEL-CAP(5) (IEA). | ||
CAP derivatives and analogs |
IC50, ng/ml |
Reactivity, % |
CAP |
0,64 |
100 |
CAP succinate |
0,29 |
218 |
CAP glucuronide |
0,50 |
128 |
CAP base |
492 |
0,13 |
Thiamphenicol |
> 1000 |
< 0,01 |
Floramphenicol |
> 1000 |
< 0,01 |
Note. BSA, GEL, CAP – resp., bovine serum albumin, gelatin, chloramphenicol. IC50 – half maximal inhibitory concentration (causes 50% inhibition of antibody binding). |
||
CAP analogues whose molecule contains a methylsulfonyl group instead of anitro group (Thiamphenicol and Floramphenicol) weren’t recognized by antibodies even at a concentration of 1000 ng/ml, so their cross-reactivity was below 0,01%. There’s a few available data about cross-reactivity of commercial test systems widely used for sanitation purposes in Russia. For example, the manufacturer of test system Ridascreen (“R-BioPharm”, Germany) declared reactivity in respect to CAP base – 0,5%, Thiamphenicol - less than 0,05%, Floramphenicol - no sensitivity; the test kit IFA-KhAF (“JSC Immunotech ", Russia) – reactivity to CAP succinate of 25%, while less than 1% to antibiotics from other groups.
Thus, the obtained antiserum to BSA-CAP(50) and solid-phase conjugates EA-CAP(25), RSA-CAP(25) or GEL-CAP(5) have allowed determination of CAP in solutions with a concentration up to 0,1 ng/ml. In multiple repetitive tests the degree of antibody binding showed a relative standard deviation of less than 0,05 indicating sustainable operation of the test system in laboratory conditions with normal fluctuations of external factors.
|
Fig. 2. Calibration curves of IEA detection of CAP with antiserum to BSA-CAP(50) from the 7th blood sample and solid phase antigen GEL-CAP(5) in the variant with simulation of background using MP, 3-fold dilution with PBST (1); in water-acetonitrile mixture, 10-fold dilution with PBST (2); in water-acetonitrile extracts of chicken meat and egg samples, 10-fold dilution with PBST (3 and 4, resp.). Denotations: |
Simulation of background by fat-free milk powder solution in PBST introduced in zero and control wells, as well as in calibration solutions suggests the possibility of testing milk diluted with PBST 3-fold (Fig. 2).
The accuracy of detection provided by the proposed test system was revealed by experiments with the introduction of CAP to milk at various doses (0,4; 2 and 10 mg/kg): on average it equaled 104% (Table 3).
| 3. Accuracy of detection of CAP in milk samples containing various introduced doses of this antibiotic in IEA with antiserum to BSA-CAP(50) from the 7th blood sample and immobilized antigen GEL-CAP(5). | ||
CAP introduced, ng/g |
CAP detected, ng/g (Х±х, n = 4) |
Accuracy of detection, % |
10 |
11,6±1,3 |
116 |
2 |
2,13±0,30 |
106 |
0,4 |
0,36±0,05 |
90 |
Note. BSA, GEL, CAP– resp., bovine serum albumin, gelatin, chloramphenicol. |
||
Experimental evaluation of test repeatability and intermediate precision against variable factors of “time”, “operator” and “equipment” had quite satisfactory results: for repeatability, relative standard deviation was 0,13; for an intermediate precision – 0,16. The analysis of milk with simulated background and dilution of an average sample with buffer 3-fold provided the lower limit of detection of CAP 0,3 mg/kg.
Analysis of 20 randomly selected samples of fresh, pasteurized and UHT milk revealed no cases of product contamination with CAP - the binding of antibodies was 90% or more. In seven tested samples of milk powder this figure ranged from 99 to 107%. However, in 2008-2009, using ELISA for screening of 106 milk samples from different regions of the country there were detected three CAP-positive samples that contained 10, 2, and 26 mg CAP/kg (12).
In foreign countries, selection of an appropriate technique for detection of CAP residues is still being discussed since 1990s (13, 14). However, it’s of no doubt the fact that approaches based on a combination of immunoassay and chromatography are inferior to ELISA in labor inputs and costs. The required minimum level of detection of CAP residues in food products in the EU was recently reduced to 0,3 mg/kg (15), so there are being performed new investigations aimed at finding the reagents satisfying this threshold of detection by ELISA (16). In Europe, USA, and Canada CAP is banned for use on animals intended for food purposes due to its specific and highly dangerous toxic effects in humans (disruption of the bone marrow and irreversible aplastic anemia). In Russia there’s also being worked through the improvement of analytical properties of antibodies to CAP that will reduce the lower limit of detection of CAP residues to <0,1 mg/kg (17).
The analysis of samples of muscle tissue and internal organs derived from chickens used in the experiment on intragastric administration of CAP has confirmed that this test system is capable of detection of this antibiotic in water-acetonitrile extracts. For individual not given the antibiotic (control), extracts of muscle tissue after 10-fold dilution with buffer didn’t show background effects (Fig. 2). For individual that received CAP (test), resulting contamination of tissues amounted to 635 ± 5 mg/kg (meat), 330 mg/kg (liver), and 250 mg/kg (heart). In 5 h after the administration of the second portion of CAP (total dose 200 mg/kg body weight), the residual amount of this antibiotic in meat and internal organs was 0,5 mg/kg, i.e. 0,25% of the administered.
Earlier, the authors reported about immunoassay of antibiotics in meat and eggs, in particular Bacitracin and Gentamicin, which is carried out after a preliminary drying and extraction with an aqueous acetonitrile at its equivalent excess relative to homogenate weighting (8, 9). Now, in the variant of water-acetonitrile extracts of chicken meat and eggs after such drying it was proved the suitability of the proposed test system (Fig. 2). In these conditions, the lower threshold of detection of CAP in the samples was 1 mg/kg (Fig. 2).
Thus, enzyme-linked immunosorbent assay (ELISA) based on polyclonal rabbit antibodies to chloramphenicol (CAP) conjugated to bovine serum albumin and immobilized antigens – CAP conjugates with egg albumin, rabbit serum albumin or gelatin – provides determination of CAP in solutions up to a concentration of 0,1 ng/ml, as well as detection of this antibiotic in milk - with a sensitivity of 0,3 mg/kg, in meat and eggs - 1 mg/kg, which allows using a simple procedure of sample preparation. This advanced ELISA-based analytical test system with highly specific detection of CAP is suitable for practical use, and it is certainly valuable for improvement of efficiency of health and sanitation control of livestock products.
REFERENCES
1. Klenova I.F. and Yaremenko N.A., Veterinarnye preparaty v Rossii. Spravochnik (Reference Book of Veterinary Medications in Russia), Moscow, 2001.
2. Kolosova A.Yu.,Immunoenzyme Assay of Antibiotics and Its Use in Drug Monitoring and Food Safety Control, Extended Abstract of Cand. Sci. Dissertation, Moscow, 1998.
3. Kolosova A.Y., Samsonova J.V., and Egorov A.M., Comparative ELISA of Chloramphenicol: Influence of Immunoreagent Structure and Application of the Method for the Inspection of Food of Animal Origin, Food Agric. Immunol., 2000, vol. 12, no. 2, pp. 115-125.
4. Sanitary and Epidemiologic Rules and Regulatios SanPiN 2.3.2.2804-10 Sanitary Requirements to Safety and Nutritive Value of Food Products, Moscow, 2010.
5. Nakane P.K. and Kawaoi A., Peroxidase-Labeled Antibody. A New Method of Conjugation, J. Histochem. Cytochem., 1974, vol. 22, no. 2, pp. 1084-1091.
6. Hermanson G.T., Bioconjugate Techniques, San Diego-New York-Boston-London-Sydney-Tokyo-Toronto: Academic Press, 1996, pp. 436-439.
7. Burkin A.A., Kononenko G.P., and Burkin M.A.,Methods of Sanitary Control of Livestock Products. I. Enzymoimmunoassay (ELISA) of Tetracyclines, S.-kh. biol., 2010, vol. 4, pp. 110-117.
8. Kononenko G.P. and Burkin A.A., Methods of Sanitary Surveillance for Livestock Production. II. Enzyme Immunoassay (EIA) of Bacitracin, S.-kh. biol., 2010, vol. 6, pp. 88-93.
9. Burkin M.A., Kononenko G.P, and Burkin A.A., Methods of Sanitary Surveillance of Livestock Production. III. Enzymoimmunoassay of Gentamicin, S.-kh. biol., 2011, vol. 2, pp. 93-98.
10. Moffat A.C., Jackson J.V., Moss M.S., and Widdop B., Clarke’s Isolation and Identification of Drugs, London: The Pharmaceutical Press, 1986.
11. Campbell G.S., Mageau R.P., Schwab B., and Johnston R.W., Detection and Quantitation of Chloramphenicol by Competitive Enzyme-Linked Immunoassay, Antimicrob. Agents Chemother., 1984, vol. 25, no. 2, pp. 205-211.
12. Burkin M.A. and Galvidis I.A., Screening of Antibiotic Contamination of Milk by Immunoassay, Molekulyarnaya meditsina, 2010, vol. 4, pp. 47-51.
13. Van de Water C. and Haagsma N., Analysis of Chloramphenicol Residues in Swine Tissues and Milk: Comparative Study Using Different Screening and Quantitative Methods, J. Chromatogr., 1991, vol. 566, no. 1, pp. 173-185.
14. Laurensen J.J. and Nouws J.F., Monitoring of Chloramphenicol Residues in Muscle Tissues by an Immunoassay (La Carte Test), Vet. Q., 1990, vol. 12, no. 2, pp. 121-123.
15. Commission Decision of 13 March 2003 Amending Decision 2002/657/EC as Regards the Setting of Minimum Required Performance Limits (MRPLs) for Certain Residues in Food of Animal Origin, Official Journal of the European Union, 2003, vol. L 71, pp. 17-18.
16. Fodey T., Murilla G., Сannavan A., and Elliott C., Characterization of Antibodies to Chloramphenicol, Produced in Different Species by Enzyme-Linked Immunosorbent Assay and Biosensor Technologies, Analytica Chimica Acta, 2007, vol. 592, pp. 51-57.
17. Samsonova J.V., Fedorova M.D., Andreeva I.P., Rubtsova M.Yu., and Ego rov A.M., Characterization of Anti-Chloramphenicol Antibodies by Enzyme-Linked Immunosorbent Assay, Anal. Lett., 2010, vol. 43, pp. 133-141.
1I.I. Mechnikov Research Institute for Vaccines and Sera, RAMS, Moscow 105064, Russia, |
Received February 24, 2012
|