УДК 636.086.2/.3:581.143.6:631.527.8
ESTIMATION OF OIL TOXICITY FOR Agrostis stolonifera L. AND BIOTECHNOLOGICAL METHOD FOR PRODUCING OF RESISTANT PLANTS
E.V. Orlova1, E.A. Gladkov1, 2, O.V. Gladkova2, A.Yu. Stepanova1
The toxic influence of the crude oil on plants and callus of Agrostis stolonifera L. was investigated. It was shown that content 1.0 % crude oil in the soil led to inhibition of growth, 5.0 % — to the death of plants. The toxicity of crude oil to calluses was higher in comparison with plants. The growth inhibition was observed after incubation of the callus in Murashige-Skoog (MS) medium containing 0.5 % of crude oil: only 52 % of Agrostis stolonifera cells was retained growth activity, and with 0.8 % of crude oil — 25 %. The incubation with 1.0 % of crude oil resulted in the arrest of the growth and the death of calluses. For further selection of oil tolerant cells the callus was cultivated in the MS medium with 0.5 % of crude oil during two subculturings, then transplanted in the medium for regeneration with the same concentration of petroleum. In total in selective conditions 19 plants were received.
Keywords: Agrostis stolonifera, cell selection, oil.
Soil pollution with crude oil and its refined products occurs at their production, dumping, unexpected spills at transportation and piping. This serious threat to the environment that can cause decline in productivity of forests and grasslands, degradation of farmlands and loss of crops’ yields. In critical situations, oil-polluted soil can transform into typical human-made desert with almost completely suppressed vital functions of the biotope.
Crude oil consists of several thousand compounds, most of which are hydrocarbons (90-95% by volume): alkanes, cycloalkanes, aromatics, asphaltenes, resins and olefins. It also contains sulfur and various trace elements. Petroleum products have a complex composition and thereby they provide complex effects on plants: a direct toxic action along with indirect effects associated with transformation of morphological, physical and chemical properties and microbiological characteristics of soil. Heavy petroleum fractions increase hydrophobicity of soil and change its physical properties, and the light fractions of petroleum hydrocarbons cause direct toxic effects (1-3). Phytotoxicity of oil-contaminated soil is manifested as significantly reduced seed germination capacity, growth inhibition in plant roots and shoots (4-7).
Phytotoxicity of crude oil has been established on several crops, including forage plants – oats, barley, clover, lupine, fescue and cock’s-foot (4). At low contamination degree, the inhibitory effect of oil reduces after 2 months to almost background level, probably due to volatility of the lighter fractions. At the high degree of pollution, heavy hydrocarbon fractions provide the long-term toxic effects associated with soil degradation. In some cases of mixed contamination, such as salinity and crude oil, only sunflower maintains its productivity, while most of agricultural grasses, crucifers, legumes and composites die (8).
The current development of oil industry can’t exclude oil contamination of the environment. This fact necessitates obtaining the plants resistant to these pollutants and able to grow on soils contaminated with oil where the recovery is needed - near oil wells, pipelines, storage facilities and petroleum refineries after the initial bioremediation (using microorganisms).
Creation of resistant crops can be performed using cell selection, which has been proved as the promising method for obtaining plants resistant to such abiotic stresses as drought, salinity, flooding and heavy metals (9-14). Despite many studies on selection of cultured cells, the authors have found the only report about assessing the toxicity of hydrocarbons (heptane and hexane) under in vitro conditions, which though didn’t include the selection of resistant cell lines and plants (15).
The purpose of this research was to develop the scheme of cell selection allowing to obtain regenerated plants and testing them for resistance to oil pollution of soil.
Technique. The object of study was creeping bentgrass (Agrostis stolonifera L.). The calli were obtained from the seeds, which were pre-sterilized with the commercial chlorine bleach “Belizna” within 30 minutes, then washed 3 times with sterile distilled water and placed on a nutrient medium Murashige-Skoog (MS) (16) containing thiamine (1 mg/l) , nicotinic acid (0,5 mg/l), pyridoxine (0,5 mg/l), meso-inositol (100 mg/l), hydrolyzed casein (500 mg/l), 2,4-dichlorophenoxyacetic acid (2,4-D) (4 mg/l), sucrose (30 g/l) and agar (7 g/l). 30 seeds were placed in each Petri dish and grown at 26 °C and relative air humidity 70 % in the light (2000 lux, daylight 16 hours). After 30 days, the calli were transplanted on the same medium containing 1 mg/l 2,4-D.
|
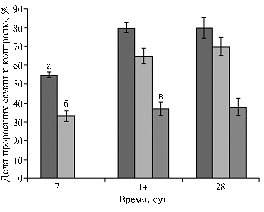
Fig. 1. Seed germination of Agrostis stolonifera L. at different contents of crude oil in soil 0,5 % (a), 0,8 % (b) и 1,0 % (c) (laboratory experiment). |
Then, the calli were regenerated on MS medium without hormones. Rooting of plants was carried out on MS medium without hormones and containing a half dose of macro- and microsalts (as recommended by the prescription) for 2-4 weeks. Time of culturing was defined depending on the degree of root development. The rooted regenerants were incubated in a greenhouse (firstly - 1 day in open culture vessels on agar, then 3 days on water) before planting in soil.
The crude oil from Nizhnevartovsk and Grozny oil fields was used in the experiments as a mixture having the following characteristics: specific density – 0,83; total hydrocarbon content – 81,2% including n-alkanes – 9,3%, isoalkanes – 7,9%, naphthenes – 11,7%, aromatic hydrocarbons – 64,0%, resins – 3,1% and asphaltenes – 4,0%. To determine the toxicity of oil to plants, it was introduced into the pots filled with soil (100 g), mixed thoroughly and the seeds were planted (20 pcs.; no less than 60 pcs. were used in each variant). A control group of plants were grown in soil without oil. To assess the toxicity of oil under in vitro conditions, the seeds were placed in Petri dishes on the agar medium containing oil at the final content of 0,5; 0,8; 1,0 and 5,0%. The oil was uniformly distributed in the agar medium: the medium was boiled, cooled to 40 °C, mixed thoroughly with the oil and poured into Petri dishes. A control - sterilized seeds grown under the same conditions without oil. Survival rate was evaluated after 7 and 14 days of cultivation.
To determine the resistance of callus tissue, it was placed on agar medium with different oil content prepared as described above. Survival rate of calli (proportion of survival calli to the total number,%) was assessed after 1 month of cultivation. The calli were incubated in Petri dishes in the light at 26 °C (2000 lux, daylight 16 hours).
The confidence interval was calculated using Student's t-test at 95% probability level.
Results. Hydrophobicity of crude oil complicates using it under in vitro conditions. In this regard, firstly, it was necessary to evaluate the suitability of using oil as a selective agent and determine the sensitivity of plants and callus cultures to this pollutant. The literature data suggest that phytotoxicity of oil depends on soil composition, its temperature and moisture along with chemical composition of the oil and belonging a tested plant to particular species (1, 6, 17, 18). For example, in maize, oil toxicity is manifested at the content of 1%, in fescue and cock’s-foot – at 5%, in clover, lupine, oat, barley – at 10%; sunflower is the most resistant to oil pollution (17%) (4). In Russia, the lowest safe content of oil products in soil amounts to 1,000 mg/kg (0,1%), the upper limit ranges from 0,1% (in permafrost tundra and taiga regions) to 1,0% (in forest-steppe and steppe regions) (19). At the same time, in areas with developed oil production, transportation and use of petroleum products, contents of these pollutants often exceed the upper safe limit. Considering this fact, this experiment was set in different variants with the oil contents varying from 0,5% to 5,0%. In the variant with 0,5% oil, seed germination was significantly suppressed - only 55% seeds germinated during the 1st week relative to control; in the variant with 0,8% oil - 33% seeds, while no seeds sprouted in soil keeping oil at the content from 1,0% to 5,0%. After 2 weeks, germination rate increased up to 80% in the variant with 0,5% oil, 65% - at the content of 0,8% and 37% - in the variant with 1,0% oil (Fig. 1).
1. Survival of calli of Agrostis stolonifera L. after the 1-month cultivation on agar medium with different contents of crude oil (laboratory experiment) |
|||
Oil content, % |
Number of calli, pcs. |
Survival rate, % |
|
total |
survived |
||
0 (control) |
100 |
100 |
100 |
0,5 |
200 |
103 |
52 |
0,8 |
200 |
50 |
25 |
1,0 |
200 |
0 |
0 |
5,0 |
50 |
0 |
0 |
This fact was most likely associated with volatility of light petrol fractions. The presence of 5,0% oil in soil (“very high pollution degree”) resulted in the complete loss of germination capacity of seeds. Germination rate of seeds sown in Petri dishes (in vitro) was 5-6 times lower than that grown in soil. In addition, under in vitro conditions (in contrast to variants with soil), the number of sprouted seeds after 14 and 28 days of cultivation remained the same as on the 7th day (possibly, the closed in vitro system prevents natural degradation of oil). At 5,0% oil content, seeds didn’t germinate nor in vitro neither in soil.
Thus, in vitro conditions were found to be more stringent than cultivation in soil, which allows using in vitro systems for selection of resistant cells. Considering the abovementioned data, the resistance of bentgrass callus cultures to oil was evaluated at its contents of 0,5; 0,8 and 1,0%.
2. Regenerative capacity of calli of Agrostis stolonifera after the 1-month cultivation on agar medium with different contents of crude oil (laboratory experiment). |
||
Oil content, % |
Number, pcs. |
|
calli (total) |
regenerated |
|
0 (control) |
100 |
90 |
0,5 |
100 |
23 |
0,8 |
100 |
10 |
1,0 |
100 |
0 |
5,0 |
100 |
0 |
During the incubation of calli on the medium with 0,5% oil, 52% calli maintained growth capacity, at 0,8% oil - 25% calli relative to control. Incubation of calli for 1 month in the media containing 1,0 and 5,0% oil resulted in stop of growth and deaths (Table 1). Incubation in selective conditions significantly inhibited their ability to morphogenesis as well. In the case with 0,5% oil in a medium, only 23% calli were capable to regenerate into plants, at 0,8% - 10% (Table 2).
|
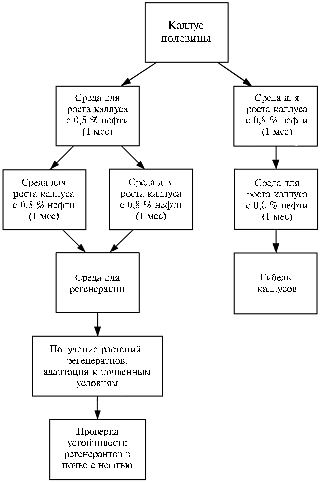
Fig. 2. The scheme of selecting the plants of Agrostis stolonifera L. resistant to oil pollution using callus cultures. |
Morphogenesis is more sensitive to stresses than growth phase, as was reported for sugar cane (11) and rice (20). Possibly, differentiation processes associated with active cell divisions require more energy and, therefore, they are become more limited during a stress. Since the main purpose of cell selection was obtaining regenerated plants, the variants with oil content of 0,5 and 0,8% were chosen as selective variants. Selection of resistant cells was performed as two stages. The first stage included incubation of the bentgrass calli on agar nutrient media containing 0,5% and 0,8% oil during 1 month. Then, among the calli grown with 0,5% oil, one part was replanted on the medium with 0,8% oil, the other part – on the medium containing 0,5% oil. The calli incubated on the medium containing 0,8% oil were re-planted into equal conditions (Fig. 2). The calli grown continuously in the medium with 0,8% oil, died after 2 months of cultivation. The calli that had been cultured for 1 month on the medium with 0,5% oil, and then on the medium with 0,8% oil, died too. Among the calli cultured in the medium with 0,5% oil for 2 months, the resistant calli were selected. These calli resulted in 19 regenerated plants, three of which formed the seeds.
The seeds obtained from the selected plants were sown on a medium containing crude oil (1,0 and 1,5%, control - the seeds from plants grown in soil without oil) and manifested germination rates equal to 70% and most similar in different variants of the experiment. In seeds of plants not subjected to the selection, these indices in the variants with 1% and 1,5% oil were, respectively, 50 and 40%. At the oil content in soil 1,5%, the survived plants from the selected group developed the stem height averaged to 5,0 cm, while not selected sprouts – only 2,5 cm (average height of plants in corresponding control groups without the toxicant – 8,0 cm). However, after 1,5 months, the height of plants grown on the oil-contaminated soil reached that of control (plants on soil without oil). Such effects can be explained by weakening of toxic effects of oil and development of oil-oxidizing microorganisms in the soil. Though, this fact didn’t change germination capacity of seeds.
Thus, for the first time the authors have assessed the toxicity of oil under in vitro conditions using callus cultures of creeping bentgrass and have developed the scheme for selection of resistant plants. The short-term methodological procedure of cell selection allows maintaining the regenerative capacity of cells and rapid obtaining of resistant plants.
REFERENCES
1. Arens V.Zh., Saushin A.Z., Gridin O.M. and Gridin A.O., Ochistka okruzhayuschei sredy ot uglevodnykh zagryaznenii (Purification of Environment from Hydrocarbon Contamination), Moscow, 1999.
2. Oborin A.A., Kalachnikova I.G., Maslivets T.A., Bazenkova E.I., Plescheva O.V. and Ogloblina A.I., Vosstanovlenieneftezagryaznennykh pochvennykh ekosistem (Recovery of Oil-Contaminated Soil Ecosystems), Moscow, 1988, pp. 140-159.
3. Zvyagintsev D.G., Guzev V.S., Levin S.V., Seletskii G.I. and Oborin A.A., Diagnostic Signs of Differences in Level of Petroleum Pollution of Soil, Pochvovedenie, 1989, no. 1, pp. 72-78.
4. Dedkov V.P. and Fominykh Ya.V., Influence of Oil Pollution on Growth and Development of Plants, in Mezhvuz. sb. nauch. tr. “Teoreticheskie i prikladnye aspekty biologii” (Compil. Sci. Works of Higher Schools: Theoretical and Practical Aspects of Biology), Kaliningrad, 1999, pp. 36-42.
5. Dedkov V.P., Grebennikov A.S. and Turkin N.I., Growth and Development of Plants on Soil Contaminated by Oil, in Mezhvuz. sb. nauch. tr. “Teoreticheskie i prikladnye aspekty biologii” (Compil. Sci. Works of Higher Schools: Theoretical and Practical Aspects of Biology), Kaliningrad, 1999, pp. 36-42.
6. Adam G. and Duncan H.J., Effect of Diesel Fuel on Growth of Selected Plant Species, Environ. Geochem. Health, 2000, vol. 21, pp. 353-357.
7. Gaskin S., Soole K. and Bentham R., Screening of Australian Native Grasses for Rhizoremediation of Aliphatic Hydrocarbon-Contaminated Soil, Int. J. Phytorem., 2008, no. 10, pp. 378-389.
8. Kireeva N.A., Bakaeva M.D. and Tarasenko E.M., Complex Biotesting for Assessment of Soil Pollution by Oil, Ecologiya i promyshlennost’ Rossii, 2004, no. 2, pp. 26-29.
9. Purnhauser L. and Gyulai G., Effect of Copper on Shoot and Root Regeneration in Wheat, Triticale, Rape and Tobacco Tissue Cultures, Plant Cell, Tissue and Organ Culture, 1993, vol. 35, pp. 131-139.
10. Dolgikh Yu.I., Larina S.N., Shamina Z.B., Zhdanova N.E. and Pustovoitova T.N., Draught Resistance of Maize Plants Obtained from Cell Lines Resistant to the Osmotic Effect of Polyethylene Glycol, Fiziologiya rastenii, 1994, vol. 41, pp. 853-858.
11. Kharinarain R.P., Guzhov Yu.L. and Dolgikh Y.I., Perspectives of the Application of Biotechnological Methods in Selection of the Sugarcane Saccharum officinarum for Resistance to Hypoxia, Izv. RAN (ser. biol.), 1996, no. 4, pp.411-421.
12. Gladkov E.A., Biotechnological Methods of Obtaining of Agrostis stolonifera Plants Resistant to Cadmium and Lead, Agrobiology, 2008, no. 3, pp. 83-87.
13. Al-Kholani Kh.A.M., Toayama V.I.M. and Dolgikh Yu.I., Development of Draught-Tolerant Corn Plants by Cellular Selection on a Medium Containing Mannitol, Biotekhnologiya, 2010, no. 1, pp. 60-67.
14. Tereshonok D.V., Stepanova A.Yu., Dolgikh Yu.I, Osipova E.S., Belyaev D.V. and Vartapetian B.B., Tolerance to Root Flooding of Wheat Plants (Triticum aestivum L.) Produced with Biotechnological Approaches, Plant Stress, 2010, vol. 4, no. 1, pp. 79-82.
15. Sergeeva L.E., In Vitro Determination of Toxicity of Paraffins, Fiziologiya i biokhimiya kul’turnykh rastenii, 2000, vol. 32, no. 4, pp. 325-328.
16. Murashige T. and Skoog F., A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures, Physiologia Plantarum, 1962, vol. 15, pp. 473-497.
17. Robson D., Knight D., Farrell R. and Germida J., Ability of Cold Tolerant Plants to Grow in Hydrocarbon-Contaminated Soil, Int. J. Phytorem.,2003, no. 5, pp. 105-123.
18. Frick C.M., Farrell R.E. and Germida J.J., Assessment of Phytoremediation as an In-Situ Technique for Cleaning Oil-Contaminated Sites, Calgary: Petroleum Technology Alliance of Canada, 1999.
19. Davydova S.L. and Tagasov V.I., Neft’ i nefteprodukty v okruzhayuschei srede (Petroleum and Products in Environment), Moscow, 2004.
20. Belyanskaya S.L., Shamina Z.B. and Kuchenko L.A., Morphogenesis of Rice Clones Resistant to Stress Factors, Fiziologiya rastenii, 1994, vol. 41, no. 4, pp. 573-577.
| 1K.A. Timiryazev Institute of Plant Physiology, RAS, Moscow 127276, Russia, e-mail: gsc@ippras.ru; 2Moscow State University for Engineering Ecology, Moscow 105066, Russia |
Received June 17, 2009 |