УДК 636/639:614.31
METHODS OF SANITARY SURVEILLANCE FOR LIVESTOCK PRODUCTION. IV. ENZYME IMMUNOASSAY OF CIPROFLOXACIN AND ITS ANALOGS
A.A. Burkin1, G.P. Kononenko1, M.A. Burkin2
On basis of polyclonal rabbit antibodies to conjugate of ciprofloxacin and bovine serum albumin, obtained as result of carbodiimidal condensation the authors developed the method of indirect competitive immune-enzyme analysis of antimicrobial substances from fluoroquinolones group. With capture conjugates, heterologous to immunogene on protein carrier (gelatin) and on method of synthesis (reaction with activated N-hydroxysuccinimide ether and formaldehyde condensation) the sensitivity of determination of ciprofloxacin and enrofloxacin is 0.1 ng/ml, and for norfloxacin and pefloxacin — 0.4 ng/ml. The possibility of the application of immune-enzyme test-systems for control of residual of these preparations in milk, meat and eggs is discussed.
Keywords: ciprofloxacin, enrofloxacin, norfloxacin, pefloxacin, immunoassay, milk, meat, eggs.
Modern synthetic antimicrobial pharmaceuticals fluoroquinolones are widely used in animal husbandry as veterinary medications. Most often these are N1-cyclopropyl-3-carboxy-6-fluoro-7-piperazinyl quinolones: ciprofloxacin (CF, R = H) and enrofloxacin (EF, R = CH2CH3) (formula shown on the left) along with the N1-ethyl-substituted analogues norfloxacin (NF, R = H) and pefloxacin (PF, R = CH3) (formula on the right):

Regulations on the total content of CF and EF residues in meat, dairy and fish products have been in force in the EU for two decades (1). The attempts to develop immunoassays for detecting them were taken abroad in 1998-2006 (2-5), which has led to a current rise in commercial production of analytical systems for testing fluoroquinolones contents in milk, meat, fish and shrimp.
The purpose of this work was obtaining immunoreagents and optimization of the indirect competitive immunoassay (ELISA) of ciprofloxacin providing test specificity and sensitivity that satisfy the established regulations on residual contents of fluoroquinolones in animal products.
Technique. The authors used N-hydroxysuccinimide (HSI), 1-ethyl-3(3-dimethylaminopropyl)carbodiimide (CDI), formaldehyde, dimethylformamide (DMF), o-phenylenediamine, horseradish peroxidase (EC 1.11.1.7) (“Sigma”, USA ), donkey anti-serum to rabbit immunoglobulin, bovine serum albumin (BSA), egg albumin (EA) and gelatin (Gel) of domestic production.
All fluoroquinolone substances were obtained from available commercial pharmaceuticals. The tablets of ReCipro 250 (“Aurobindo Farma Ltd.”, India) containing CF hydrochloride monohydrate were carefully pounded and added with methanol, intensely mixed, left for 1 day and then filtered; the filtrate was dried at 40 °C under vacuum and the residue was weighted. The identity of the isolated substance was proved by UV spectroscopy. The product purity was assessed by comparing its concentration in the solution using two methods - gravimetric and spectrophotometric, and then it was used in conjugation reactions with proteins. To extract EF, CF and PF, the medications (tablets or powder) Enroxil (“KRKA”, Slovenia), Nolicin (“KRKA”, Slovenia) and Abaktal (“Lek”, Slovenia) were added with DMFA at the quantities corresponding to amount of each substance (ml/mg), intensely mixed, left for 1 day and filtered. Aliquots of the filtrates were diluted with water and then the concentration was measured by UV spectroscopy. The obtained solutions were used to assess cross-reactivity of antibodies. CF calibration solutions were prepared from the ciprofloxacin for injections (“Wockhardt Ltd.”, India) considering the nominal values of CF equivalents.
To synthesize the conjugates BSA-CFa (with 100-fold molar excess of hapten) and Gel-CFa (with 25-fold molar excess of hapten) via the reaction of activated HSI-esters, the samples of HSI (2 mg; 17 umol) and CDI (3,3 mg; 17 umol) were added to 1,08 ml CF solution (4 mg; 12,1 umol) in DMFA. After 24 hours of stirring at room temperature, 0,89 ml reaction mixture was dropwise added to BSA (7 mg; 0,1 umol) and 0,11 ml - to Gel (8 mg; 0,05 umol) (both were taken as dissolved in 2 ml of 0,05 M carbonate-bicarbonate buffer (pH 9,5), then stirred at room temperature for 2 h and dialyzed.
To obtain the conjugates BSA-CFc (with 170-fold molar excess of hapten) and EA-CFc (with 60-fold molar excess of hapten) via carbodiimide condensation, the solutions of BSA (5 mg; 0,07 umol) and EA (4 mg; 0,1 umol) in 2 ml water were added with CF (4 and 2 mg, respectively, or 12 and 6 umol) and CDI (30 and 25 mg, respectively, or 156 and 130 umol). The reaction mixtures were stirred for 4 h at 30 °C, then stirred for 20 hours at 4 °C and dialyzed.
BSA-CFf was synthesized (with 100-fold molar excess of hapten) via formaldehyde condensation: BSA solution (5 mg; 0,07 umol) in 1,5 ml water was added with CF (7 umol) and 0,3 ml 37% formaldehyde solution (3690 umol), the mixture was stirred at 30 °C for 3 hours and dialyzed. The dialysis was performed during 2 days using 3 changes of 1000-fold volumes of 0,5% NaCl. The products of dialysis were added with equal volumes of glycerol up to the content 1 mg/ml (for protein) and then stored at –10 °C.
Immunization procedures and the competitive ELISA were performed as described previously (6). The development of immune response in animals was analyzed from the reaction of the serum from each blood sampling with immobilized conjugate BSA-CFf. After the stabilization of antibody titers, the sera were tested with other immobilized conjugates for binding and inhibition in the competitive analysis with CF. Cross-reactivity of antibodies against EF, NF and PF was assessed using the solid-phase antigens whose upper limit of the linear segment on a calibration curve corresponded to the lowest concentration of free CF. Cross-reactivity of antibodies was defined as the ratio of the CF solution concentration causing 50% inhibition of binding to a solid phase (IC50) relative to corresponding concentrations of other free fluoroquinolones, expressed as a percentage. UV spectra were recorded on the device Hitachi-557 (Japan). ELISA was performed on Dynatech microplates with registration of results on the photometer Dynatech MR5000 (Germany).
The tested samples of cow's milk, chicken, chicken meat, by-products and eggs were obtained from the network market of Moscow and analyzed using the optimized version of test system. Preparation of samples was performed as described previously (7). The milk was diluted with phosphate-buffered saline (pH 7,5) containing 0,01 M Na2HPO4; 0,14 M NaCl and 0,05% Tween 20 (PBS-T). Homogenates of meat, by-products and eggs were weighed, dried on aluminum plates by hot drying fan, extracted with acetonitrile-water mixture (taken at the volume ratio 84:16) for 14 hours with intensive stirring at the beginning and end of the extraction; the extracts were 10-fold diluted with PBS-T.
Results. The nominal content of CF equivalents in the used officinal drug (“Wockhardt Ltd.”, India) was confirmed by the data obtained at comparing the specific absorption (A, 1%, 1cm) at l = 275 nm in the solution prepared from it by the 200-fold dilution with water and the corresponding values given by I.V. Titov at al. (8).
| 1. Characteristics of UV-absorption of prepared aqueous solutions of fluoroquinolones | |||
Substance |
Location of absorption maxima |
Ratio of intensities of absorption maxima |
|
λ1, λ2, λ3, nm |
А1/А2 |
А2/А3 |
|
CF (8) |
275, 318, 328 |
3,0 |
1,06 |
EF |
272, 321, 330 |
2,7 |
1,03 |
NF |
273, 320, 330 |
2,8 |
1,05 |
PF |
273, 320, 330 |
3,0 |
1,05 |
Note. CF, EF, NF and PF – respectively, ciprofloxacin, enrofloxacin, norfloxacin, pefloxacin. |
|||
|
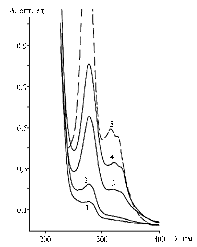
Fig. 1. UV-absorption spectra of bovine serum albumine (BSA) (1), conjgates BSA-CFa (2), BSA-CFc (3), BSA-CFf (4) and ciprofloxacin (CF) (5). CFa, CFc, CFf – CF binded to protein via the reaction of, respectively, activated ester, carbodiimide condensation and formaldehyde condensation. |
In aqueous solutions of CF hydrochloride derived from the tableted medication, the location of UV absorption maxima and the ratio of their intensities corresponded to those established in the reference samples (Table 1). For EF, NF and PF, these parameters were almost identical to those of CF with no significant differences.
From the full UV spectrum of CF (8) there were calculated molar extinction coefficients for the absorption maxima at l = 275 nm (e = 37480), l = 318 nm (e = 12490) and l = 328 nm (e= 11760), which then were used to define more exactly the concentrations of analytic solutions of fluoroquinolones. Upon these data, there was confirmed the sufficient purity degree of the dry CF sample further used for conjugation with proteins. This sample was used to prepare a solution with CF content 1 mg/ml (found gravimetrically), and after a 100-fold dilution with water the concentration amounted to 11,6 ± 0,3 ug/ml (from spectral analysis).
The fact of obtaining the conjugates of BSA with CF in three different condensation reactions was confirmed by spectral analysis (Fig. 1). Along with the increase in absorbance at l = 280 nm, the spectra of the reaction products revealed a new peak at l = 325-328 nm peculiar to hapten. In contrast to carbodiimide condensation, the reaction of activated N-hydroxysuccinimide ester at a 24-hour interaction has resulted in a lower yield (lower epitope density of the resulting conjugate). Even the lower result was obtained after the formaldehyde condensation with a 100-fold molar excess. The complications in these last two reactions can be explained by steric obstacles, because they involved the basic structure of hapten molecule rather than distant functional groups.
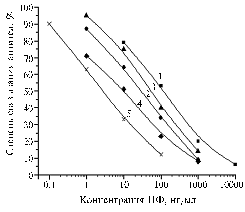 |
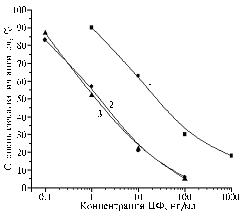
Fig. 2. The degree of antibody binding to BSA-CFa obtained at the 1st-5th blood samplings (1-5) with the solid-phase antigen BSA-CFf at the content 0,5 ug/ml at presence of CF (designations – see Fig.1). |
The immunogens were the conjugates BSA-CFa and BSA-CFc. The first isolated antiserum to BSA-CFa at interaction with solid-phase BSA-CFf has allowed detecting CF content up to 10 ng/ml, further sensitivity increased by an order, and after the 5th blood sampling it became possible to measure less than 1 ng/ml (Fig. 2).
With all the immobilized antigens, antibodies identified predominantly CF (Table 2). The antigen Gel-CFa was homologous to the immunogen by synthesis method; it showed high cross-reactivity with the three closest analogs of CF, which declined with rise of structural differences: for EF, NF and PF, respectively 68, 41 and 24%.
2. Cross-reactivity of antibodies to BSA-CFa in reaction with fluoroquinolones at interaction with different immobilized antigens |
||||||
Free antigen |
Immobilized antigen (С = 0,05 ug/ml) |
|||||
Gel-CFa |
EA-CFc |
BSA-CFf |
||||
% |
IC50, ng/ml |
% |
IC50, ng/ml |
% |
IC50, ng/ml |
|
CF |
100 |
5,9 |
100 |
2,0 |
100 |
0,9 |
EF |
68 |
8,7 |
23 |
8,8 |
31 |
2,9 |
NF |
41 |
14,5 |
21 |
9,6 |
8 |
17,0 |
PF |
24 |
24,6 |
14 |
14,5 |
6 |
14,0 |
Note. CF, EF, NF, PF – respectively, ciprofloxacin, enrofloxacin, norfloxacin and pefloxacin; BSA, Gel, EA – respectively, bovine serum albumin, gelatin, egg albumin; CFa, CFc, CFf – CF binded to protein via the reaction of, respectively, activated ester, carbodiimide condensation and formaldehyde condensation. |
||||||
|
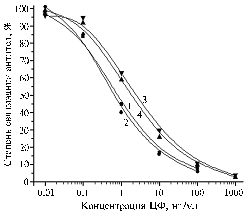
Fig. 3. The degree of antibody binding to BSA-CFc obtained at the 1st-3rd blood samplings (1-3) with the solid-phase antigen BSA-CFf at the content 0,15 ug/ml at presence of CF (designations – see Fig.1). |
Interestingly that similar properties have been reported earlier for the test system based on antibodies to BSA-CFa and the immobilized conjugate of CF with human serum albumin: relative competitive ability of EF was 69,8%, NF – 44,6% (3)
The immobilization of EA-CFc conjugate synthesized by CDI-condensation weakly affected test specificity; this just slightly reduced recognition of the same three compounds compared with CF. The analytic capabilities of antibodies to BSA-CFa changed relative to the BSA-CF product of formaldehyde condensation. The pronounced cross-reactivity was observed only with EF (31%), while no more than 6-8% with other substances. Immobilization of BSA-CFf provided the greatest test sensitivity, which can be explained by its structure apparently heterologous to the immunogen since a different binding site of the molecule (most likely through protons of the aromatic ring).
The introduction of BSA-CFc resulted in pronounced development of immune response as well. In the 2nd and 3rd sera, the antibody titers reached 1:25 000 providing in the competitive analysis with heterologous solid-phase BSA-CFf the confident determination of CF up to 0,1 ng/ml (Fig. 3). During pre-test of the antibodies with homologous EA-CFc, the extremely high binding degree without inhibiting CF occurred even at a content 1000 ng/ml, so the further evaluation of this antiserum was carried out using only two solid-phase antigens (Table 3).
3. Cross-reactivity of antibodies to BSA-CFc in reaction with fluoroquinolones at interaction with different immobilized antigens |
||||
Free antigen |
Immobilized antigen (С = 0,15 ug/ml) |
|||
Gel-CFa |
BSA-CFf |
|||
% |
IC50, ng/ml |
% |
IC50, ng/ml |
|
CF |
100 |
0,8 |
100 |
0,6 |
EF |
114 |
0,7 |
120 |
0,5 |
NF |
32 |
2,5 |
38 |
1,6 |
PF |
42 |
1,9 |
33 |
1,8 |
Note. See Table 2. |
||||
Antibodies to BSA-CFc manifested separate group specificity to N-cyclopropyl fluoroquinolones (CF and EF) and N-ethyl substituted fluoroquinolones (NF and PF) and the sufficiently high test sensitivity with both immobilized antigens: IC50 for all compounds were less than 3 ng/ml (Table 3).
Similar specificity properties were established in antibodies induced by CDI-conjugates of other fluoroquinolones. Thus, most of monoclonal antibodies induced by the conjugate of sarafloxacin showed the obviously similar detection of hapten and N-methyl analogue of difloxacin (9). Antibodies to the conjugate of EA and NF synthesized by CDI-condensation were found to have a much greater cross-reactivity with enoxacyn (the structurally close substance of a naftridon series) than with hapten (10). It is possible that, in contrast to the reaction via HSI-activated carboxyl group, CDI-condensation contributes to a special orientation of hapten on a protein when the carrier’s distal part contains the varying degrees of common and distinct structural fragments of the fluoroquinolone. However, the discussed possibility of conjugation of 7-amino piperazinyl fluoroquinolones via the secondary amino group of piperazine ring (10) is doubted by the authors.
|
Fig. 4. Calibration curves for CF (1), EF (2), NF (3) and PF (4) at the interaction of antiserum to BSA-CFc from the 3rd blood sampling with the solid-phase antigen Gel-CFa in PBST: Gel – gelatin. Other designations – see Fig. 1. |
Calibration curves for CF, EF, NF and PF with immobilization of Gel-CFa (Fig. 4) show that carrying out the analysis in buffer solution, test sensitivity to CF and EF was 0,1 ng/ml, while to NF and PF – 0,4 ng/ml . Immobilization of BSA-CFf provided similar characteristics of detecting these fluoroquinolones, so both these antigens can be equally used as reagents in test systems.
Validation experiments confirmed suitability of the developed test systems for the analysis of milk. In the cow’s milk 3-fold diluted with PBS-T, the competitive analysis with immobilized Gel-CFa and CF solutions (10; 1 and 0,1 ng/ml) provided binding of antibodies amounted to, respectively, 18±1, 46±2 and 81±4%, which values were close to those obtained in PBS-T – respectively, 17±1, 45±2 and 83±4%. Under the described conditions, sensitivity of detecting CF and EF in the milk was 0,3 ug/ kg, NF and PF – 1,2 ug/ kg.
4. The binding degree (%) of antibodies to BSA-CFc in different poultry products (samples extracted with water-acetonitrile mixture) |
|||
Sample |
CF, ng/ml |
||
0,1 |
1 |
10 |
|
Control (extractant) |
84±4 |
45±2 |
17±1 |
Chicken breast muscle |
86±6 |
46±3 |
18±2 |
Chicken thigh muscle |
82±5 |
47±4 |
17±1 |
Chicken liver |
82±6 |
42±3 |
16±1 |
Chicken stomachs |
81±4 |
44±3 |
16±3 |
Chicken hearts |
85±7 |
46±2 |
18±2 |
Chicken eggs |
83±2 |
47±4 |
18±3 |
When performing the analysis in media with the extractant (the buffer solution added with 10% acetonitrile-water mixture taken at the volume ratio 84:16), test sensitivity didn’t reduce.
In water-acetonitrile extracts of the tested samples of poultry products (meat, by-products, eggs), no background effects were observed (Table 4).
Under these conditions, in chicken meat and eggs the lower limit of detecting CF and EF was established at 1 ug/kg, NF and PF – 4 ug/kg. Considering the maximum allowable levels of CF and EF residues in meat and by-products equal to 100-300 ug/kg in the EU (1), the developed test system ensures test sensitivity with the exceeding capacity of two orders. The EU has banned using fluoroquinolones in treatment of poultry producing eggs for food purposes; therefore, its quite important to have the highly sensitive indication of these medications in foodstuff.
Thus, the immunoassay test system based on polyclonal rabbit antibodies to the conjugate of ciprofloxacin and bovine serum albumin synthesized by carbodiimide condensation allows the group detection of N-cyclopropyl fluoroquinolones (ciprofloxacin and enrofloxacin) and N-ethyl fluoroquinolones (norfloxacin and pefloxacin). The developed test system can reliably measure residual contents of these substances in milk, meat, eggs, and it can be recommended for the use in health monitoring for animal products.
REFERENCES
1. Council Regulation (EU) N 37/2010 of 22 December 2009 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin, Official Journal of European Union, 2010, vol. L15, pp. 1-72.
2. Mellgren C. and Sternesjo A., Optical Immunobiosensor Assay for Determining Enrofloxacin and Ciprofloxacin in Bovine Milk, J. AOAC International, 1998, vol. 81, no. 2, pp. 394-397.
3. Duan J. and Yuan Z., Development of an Indirect Competitive ELISA for Ciprofloxacin Residues in Food Animal Edible Tissues, J. Agricult. Food Chem., 2001, vol. 49, pp. 1087-1089.
4. Watanabe H., Satake А., Kido Y. and Tsui A., Monoclonal-Based Enzyme-Linked Immunosorbent Assay and Immunochromatographic Assay for Enrofloxacin in Biological Matrices, Analyst, 2002, vol. 127, no. 1, pp. 98-103.
5. Lu S., Zhang Y., Liu J., Zhao C., Liu W. and Xi R., Preparation of Anti-Perfloxacin Antibody and Development of an Indirect Competitive Enzyme-Linked Immunosorbent Assay for Detection of Perfloxacin Residue in Chicken Liver, J. Agricult. Food Chem., 2006, vol. 54, pp. 6995-7000.
6. Burkin M.A., Enzyme-Linked Immunosorbent Assays of Fluoroquinolones with Selective and Group Specificities, Food Agricult. Immunol., 2008, vol. 19, no. 2, pp. 131-140.
7. Burkin M.A., Kononenko G.P. and Burkin A.A., Methods of Sanitary Surveillance for Livestock Production. III. Ensymoimmunoassay of Gentamicin, S.-kh. biol., 2010, no. 2, pp. 88-93.
8. Titov I.V., Dorofeev V.L. and Arzamastsev A.P., The Use of UV-Spectrometry Method to Authenticate Fluoroquinolone Drugs, Vest. VGU (ser. khim., biol., farm.), 2004, no. 2, pp. 264-269.
9. Holtzapple C.K., Buckley S.A. and Stanker L.H., Production and Characterization of Monoclonal Antibody against Sarafloxacin and Cross-Reactivity Studies of Related Fluoroquinolones, J. Agricult. Food Chem., 1997, vol. 45, pp. 1984-1990.
10. Bucknall S., Silverlight J., Coldham N., Thorne L. and Jackman M., Antibodies to the Quinolones and Fluoroquinolones for the Development of Generic and Specific Immunoassays for Detection of These Residues in Animal Products, Food Addit. Contam., 2003, vol. 20, pp. 221-228.
1All-Russia Research and Development Institute |
Received March 23, 2011 |