ÓÄÊ 636.03:619:614.876
ABOUT ESTIMATING OF RADIATION EXPOSURE AND EFFECTS OF LATENT PATHOLOGY DURING ACUTE RADIATION DISEASE IN FARM ANIMALS
N.N. Isamov, N.N. Isamov (Jr)
In experiments on farm animals the authors estimated the effects of external γ-radiation in doses at 2-7 Gr. They studied the level of health in animals at the period of «iodine hazard» after the Chernobyl accident. It was shown, that the internal organs and tissues of animals was subject of influence by γ-radiation from radionuclides incorporated with fodder. The so-called individual radiosensitivity of animals was primarily connected with latent pathologies, both infective and non-infective, a small percentage of which is often observed among clinically healthy animals used for the dose-effect estimation.
Key words: γ- and X-ray radiation, agricultural animals, individual radiosensitivity, latent pathologies, half-lethal doses.
When studying the external ionizing radiation effects in animals, lethal doses of radiation exposure are considered to be the most informative criteria, whose effectiveness is estimated for the period from 10 to 60 days. The obtained result depends on several factors including a spatial distribution of radiation sources. For the X-ray machines, radioactive substances (60Co, 137Cs etc.) and the collimated g-radiation in experimental nuclear blasts, this is one-sided and often dotty (x-ray tube) radiation of different hardness.
Animal species differ in their radiosensitivity. Many authors also believe that there are significant differences even within species (1) while the nature of this fact still remains poorly understood (2).
1. Half-lethal dose of radiation exposure in acute radiation poisoning (1) |
|
Animal species |
LD50/30, Gy |
Rabbit |
7-8 |
Rat |
5-6 |
Mouse |
4-6 |
Sheep |
3-5 |
Monkey |
3-5 |
Dog |
2,5-4 |
Pig |
2,5-3,5 |
Guinea pig |
2-3 |
This work presents the authors’ attempt to analyze the concept of individual radiosensitivity upon the results of 30-year experiments on γ-irradiation of farm and laboratory animals. First of all, the system of literature data and own results should clearly distinguish the acute and subacute forms of radiation sickness, that is, to use the criteria LD50/30 with no combination of data obtained for this dosage with the results obtained for LD50/45 and LD50/60.
The same methodological approach should be applied when clarifying LD100. In some cases, the criterion of mortality can’t be the representative test owing to generalization of experimental data obtained from the use of ionizing radiation sources without regard to their physical parameters and timing of monitoring of animals’ death. So, the presented summary data (1) are characterized as "... the individual variability of radiosensitivity in some animals." At the same time, the difference in LD50/30 within a certain specimen of sheep and monkey amounted to 1,7 times (Table 1).
Possibly, some of the data in Table 1 were obtained from re-calculation of exposure dose into an absorbed dose, as well as some other factors not accounted for objective reasons could take place. As a result, LD50/30 for most of the animals differed within species in about 1,5 times. Meanwhile, it has been shown that the multiplicity of differences in biological effect depends on spatial distribution of irradiation. Thus, the comparison of one-sided and three-dimensional irradiation of dogs has shown that the effects of dosage ranged 100 - 600 R decreased in 2,0-1,5 times (3). The similar facts were observed for other species, particularly, for odd-toed ungulates. The half-lethal dose (LD50/60) for donkey equaled 545 R (4), and the average LD50/30 obtained by different researchers was 585 R for donkey and 650 R - for horses (2) (though, the author of the cited monograph doubted the correctness of LD100/30 for horses). Experimentally obtained exposure doses (R) recalculated by other authors into average absorbed doses (Gy) in some cases imitate a wide range of doses within a species of donkey and horse (Table 2). Recalculation of half-lethal exposure dose for horses to an average absorbed dose resulted in 3,5-4,0 Gy (5).
| 2. Half-lethal and lethal doses of radiation exposure for odd-toed ungulates | ||||
Animal species |
Radiation sourse |
LD50/30, R |
LD100/30, R |
Reference |
Donkey |
X-ray |
650-780 |
|
Rybak P.Ja., 1959 |
|
182Òà |
545à |
|
(4) |
X-ray and γ-ray |
585 |
750 |
(2)b |
|
X-ray and γ-ray |
2,1-5,5 Gy |
7,5 Gy |
(5)b |
|
Horse |
X-ray and γ-ray |
650 |
1150 (?) |
(2)b |
|
γ-ray |
670 |
|
Bell M., 1971 |
X-ray and γ-ray |
3,5-4,0 Gy |
5,0-6,5 Gy |
(5)b |
|
γ-ray |
510 |
|
Adagamov V.S., 1988 |
|
137Ñs |
5,0 Gy |
7,5 Gy |
Own data |
|
Note: a — LD50/60; b — overview data. |
||||
The presented data confirm the necessity of clarifying the question of so-called individual radiosensitivity within animal species. In the authors’ studies, animals were subjected to strictly similar parameters. Volume (bilateral) irradiation was provided by 8 blocks of the mobile device Gamma-panorama 1 (Russia) or the stationary gamma irradiator GUZh-24 (Russia) containing 137Cs as a source of radiation in both devices. Monitoring of doses under the given dosage rate and the uniformity of g-radiation was carried out by thermoluminescent dosimeters LIF (Russia). The dosimeter VAJ-18 with an ionization chamber VAK-253 (Germany) was used to control the providing of γ-radiation dose rate 1 Gy/h. The animals were irradiated by doses from 2 to 7 Gy. The volumetric dosage field for horses, cattle, sheep and pigs was a parallelepiped of 2,0 x 1,8 x 0,6 m. To irradiate the large animals, they were placed into wooden stands individually, while sheep and immature pigs - 4 individuals in 2 rows one above the other in wooden cages. The thermoluminescent dosimeters were placed across the entire surface of radiation field at a distance of 50 cm from each other. A non-uniformity of animal body irradiation did not exceed ± 10%.
Animal organism is constantly exposed to adverse impact of etiologic agents: for infections – these are viruses and bacteria, for invasions - parasites in the gastrointestinal tract, lungs and other organs and tissues, other forms of pathogens, for non-contagious diseases – disturbances of feeding conditions, housing, natural and climatic factors. The organism reactions to pathogen impact are expressed as a change in immune responsiveness connected with general immunity. In extreme situations, ionizing radiation can be the leading factor affecting immunoreactivity.
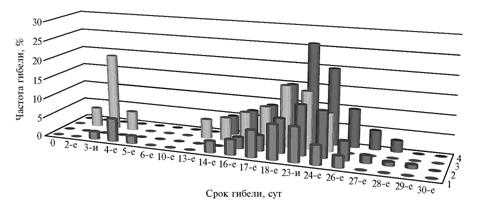
Ionizing radiation causes a pathological effect at both external body exposures and during the contact with radioactive substances brought with food into the gastrointestinal tract. An external irradiation, especially its sublethal and lethal doses, reduces natural resistance and thereby provokes or modifies the development of latent disease. Under the influence of radiation factor, animal susceptibility to exogenous infection increases. Chronic diseases (tuberculosis, brucellosis) develop into an acute form, and a response to an allergen becomes altered (6). Besides, radiation can provoke latent infections. At the experimental γ-irradiation of adult horses showing a positive reaction for carrying a latent leptospirosis infection (microagglutination test with serogroups of Leptospira grippotyphosa, L. icterohaemorrhagiae, L. tarassovi, L. pomona), a part of these horses died in the initial period of acute radiation sickness even at the minimum lethal dose (3 Gy) (Fig. 1), which is considerably earlier above the animals with a negative result of the test (7). At the same time, a half-lethal dose (LD50/30) for clinically healthy horses was 5 Gy.
|
Fig. 1 Dynamics of deaths in clinically healthy horses and the horses carrying latent leptospirosis infection depending on doses of γ-radiation: 1 and 3 – the horses-carriers of the latent infection at doses of 3-5 and 5-7 Gy, respectively; 2 and 4 – the clinically healthy horses at doses of 3-5 and 5-7 Gy, resp. Note: abscissa – Date of death, day (of exposure); denotation under the axis – 0, 2nd 3rd, 4th, 5th, 6th, 10th, 13th, 14th, 16th, 17th, 18th, 23rd, 24th, 26th, 27th, 28th, 29th, 30th. Ordinate – Death rate, % |
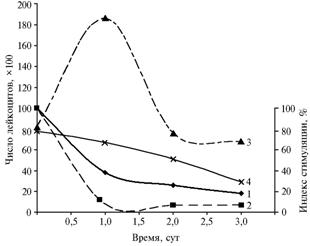
The horses-carriers of latent infection died during the 1st week after irradiation showed the following clinical symptoms of acute leptospirosis: sharply expressed apparent and real colic, the development of profound collapse and other clinical manifestations not peculiar to the development of acute radiation sickness in healthy horses and other farm animals. In addition, the latently sick horses demonstrated deviations in some indicators of immunity status. After the irradiation, it has been detected an abortive release of leukocytes in healthy animals contrasting to absence of similar reaction in the horses test-positive to leptospirosis. Latent infection weakened body resistance which contributed to the significant decrease in functional activity of T-lymphocytes (Fig. 2).
At the dose of 5 Gy, the number of leukocytes in the latently sick animals decreased by 10,5% than the initial. Functional activity of T-lymphocytes decreased by 7% (8). The optical density of neutrophils raised in 2,5-5,5 times, whereas in healthy horses it increased in 1,5 - 2,0 times relative the non-irradiated control. A part of the latently sick horses died in 1 - 5 days instead of 15 - 25 (the data on healthy animals’ deaths at a half-lethal dose of g-radiation) (9).
|
Fig. 2 Dynamics of leukocytes number and stimulation index (S.I.) of T-lymphocytes in horses after γ-irradiation at a dose of 5 Gy: 1 and 2 – S.I. of healthy and latently sick horses, respectively, 3 and 4 – number of leukocytes in healthy and latently sick horses, resp. Note: abscissa – Date, days; ordinate (right) – Stimulation index, % ordinate (left) – Number of leucocytes?100 |
After the Chernobyl accident (1986), there were inspected the farm animals shepherded in the territory contaminated with radionuclides; no sensitization to specific allergens (tuberculin, brucellin, mallein) was observed, while the epizootic situation in agriculture was fairly stable. The exceptions were the delayed effects of internal irradiation of thyroid gland with 131I eliminated in the "period of iodine danger”, which caused an injury of thyroid tissue (up to its complete necrosis and resorption) (10). As a result of this, the weak animals from a damaged flock of sheep (consumed lots of radioactive iodine when feeding near the borderline with 30-kilometer zone in the 1st month after the accident) in a distant period (2 years) got sick with hard pasteurellosis to death.
The modifying effect of γ-irradiation was also observed for helminthosis. The autors studied characteristics of radiation impact against a background of natural ascariasis infection of animals (immature pigs with body weight 25-30 kg) from disadvantaged farms. The invasion wasn’t clinically manifested in the purchased pigs, but the additional hematological tests revealed a tendency to leukocytosis and eosinophilia in some of the animals. The number of leukocytes in the animals with suspected Ascaris helminthosis amounted to 27,8x103/mkl, in clinically healthy ones - less than 16,0x103/mkl. After the γ-irradiation with half-lethal dose (4 Gy), the first ones demonstrated the transition of leukocytosis into a severe leucopenia, and 1 day later the number of leukocytes reduced by 20% of the initial. At the same time, in healthy pigs irradiated with higher doses, this value decreased only by 40%) (11). The animals with expected helminthosis were dying first, and an autopsy of these pigs revealed adult forms of Ascaris suum in their small intestine.
Modifying and provoking effect of γ-irradiation has been also established for chronic non-contagious diseases occurring without clinical manifestations. Usually, in farms, these are not fully cured calves, lambs and adult sheep recovered after bronchopneumonia or other forms of lung diseases (10). Ionizing radiation weakens immune system, which consequently aggravates the process of non-contagious diseases (12).
The development of pneumonia at radiation sickness is usually caused by activation of microbial flora located in animal’s respiratory organs or artificially introduced into the lungs (13). Experimental γ-irradiation with minimum lethal dose (3 Gy) of calves the convalescent of bronchopneumonia contributed to a profound shift in adaptation and immunoreactivity of their organisms with subsequent decompensation accompanied with a sharp and significant increase in death rates (12).
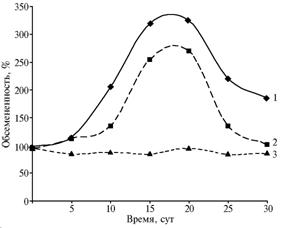
The degree of microbial colonization in farm animals’ upper respiratory tract depends on both the stage of acute radiation sickness and physiological state of an organism. In authors’ experiments on clinically healthy farm animals, the increase of bacterial number in the mucosa of nasal cavity and the change in microflora composition were observed in latent period of the disease, and the maximum colonization was detected in a midst of radiation sickness (15th-20th day). Irradiation of pregnant ewes with half-lethal or near doses resulted in more lasting and higher colonization of opportunistic microflora in the upper respiratory tract mucus membrane (Fig. 3). At LD50/30 , death rates in the pregnant ewes was 66-80% instead of 50%.
|
Fig. 3 Dynamics of microbial colonization in the nasal mucosa of pregnant ewes and non-pregnant sheep subjected to LD50/30 of γ-radiation: 1 – pregnant ewes, 2 – non-pregnant sheep, 3 – control (not irradiated sheep). Note: Abscissa – Time, days Ordinate – Microbial colonization, % |
The role of opportunistic microflora as aggravating factor in acute radiation sickness was confirmed in specific experiments on amicrobic mini-pigs (14). The authors have established the LD50/30 for 2-weeks-old amicrobic piglets equal to 3,38 Gy (338 R), while 2,86 Gy (286 R) for piglets of ordinary type, therefore, the "aseptic" pigs happen to be more resistant to ionizing radiation. The γ-irradiation of mice with latent salmonellosis have also shown that carrying latent infection aggravates the process of radiation sickness (15). In these experiments, white mice were administered per os with Salmonella strains not causing the disease prior the radiation exposure, but after the exposure this contributed to complication of acute radiation sickness. The other researchers have shown that guinea pigs with latent pasterellosis have a lower radioresistance than the animals free from these bacteria (16).
Climatic factors, particularly temperature, are of no less importance for the development and course of acute radiation sickness in farm animals. In the Samarkand region, it has been established that γ-irradiation (half-lethal dose 4 Gy) of sheep during a summer heat was followed by aggravation of acute radiation sickness with highly expressed clinical symptoms (hemorrhagic flow from the nasal cavity) (11). In central Russia, it wasn’t observed such clinical manifestations in summertime even at LD100/30.
Pathological effects of b-radiation occur in animals’ internal organs from the radionuclides administered into digestive tract with food. Among the cattle evacuated from Chernobyl which earlier had been grazed for few months in the 30-kilometer area around the Chernobyl power plant, the authors observed partial or complete suppression of thyroid function and degeneration of the thyroid gland (in the I decade of May 1986, the local content of 131I in grass was 7,4 MBq/kg, or 2x10-4 Ku/kg and above) (17). The proportion of 131I reached 42-46% relative the sum radionuclides content in the grass of some areas of Khoyniki region. The average value of emergency radionuclides precipitation in the Chernobyl region was 20% (18). The absorbed dose in the thyroid gland of the cattle, according to various estimates, ranged from 227 to 600 Gy, which resulted in hypothyroidism various degrees up to an athyreosis detected by diagnostic inspections of these animals performed in 2 years after the accident using tracer amounts of 131I (12).
Thus, the authors’ results suggest that ionizing radiation cause in farm animals both provocative and modifying influence on latent (clinically not manifested) diseases of different etiologies. In this regard, individual radiosensitivity within a species in clinically healthy animals is primarily determined by the presence of hidden changes in homeostasis caused by various etiological factors. In addition, when using the criterion dose-effect both within a species and at interspecific level, one should consider the spatial distribution of exposure (one-, two-sided) for large laboratory and all farm animals. Peculiarities of the multi-etiology pathology against a background of radiation lesion necessitate the use of differentiated prevention methods and cure measures.
An external γ-irradiation was found to be the most dangerous (starting from sublethal doses close to minimum lethal), while the danger of radioactive substances brought with food and systemic lesion manifestations primarily depend on radionuclide’s tropism and quantity. Therefore, carrying leptospirosis and other latent infectious diseases requires preventive measures including using effective antibiotics. For helminthoses, the deworming of immature abdominal helminthes is recommended. The animals recovering from non-communicable diseases should be treated with combined therapy including the use of anabolic steroids, antibiotics and immunomodulators.
REFERENCES
1.Radionuklidnoye zagryazneniye okruzhajuschei sredy i zdorov’e naseleniya(Radionuclide Contamination of the Environment and People’s Health), Vasilenko I.Ya. and Buldakova L.A., Eds., Moscow, 2004.
2. Vokken G.G., Veterinarnaya radiologiya (Veterinary Radiology), Moscow – Leningrad, 1964.
3. Besyadovskii R.A., Ivanov K.V. and Kozjura A.K., Spravochnoye rukovodstvo dlya radiobiologov (Reference Manual for Radiobiologists), Moscow, 1978.
4. Brown D.G., Johnson D.F. and Cross F.H., Late Effects Observed in Burros Surviving Eternal Whole-Body Gamma Irradiation, Radiation Res., 1965, vol. 25, pp. 574-585.
5. Belov A.D., Kirshin V.A., Lysenko N.P. et al., Radiobiologiya (Radiobiology), Belov A.D., Ed., Moscow, 1999.
6. Kruglov V.T., Dermal Allergic Responses in Farm Animals at Radiation Sickness, in: Mat. 3-j nauch. konf. “Ispol’zovaniye radioizotopov I ionizirujuschikh izluchenij v veterinarii i zhivotnovodstve (Proc., III Sci. Congress “The Use of Radioactive Isotopes and Ionizing Radiation in Veterinary and Animal Husbandry”), part 2, Moscow, 1972, pp. 47-48.
7. Isamov N.N., Sarukhanov V.Ya. and Kozlov V.A., The Modifying Influence of Irradiation on Immunoreactivity of an Organism and Latent Infections, in: Mat. konf. “Biosfera i chelovechestvo” (Proc., the Congress “Biosphere and Human Civilization”), Obninsk, 2000, pp. 215-219.
8. Isamov N.N., Shevchenko T.S. and Eliseeva I.V., Radiobiological Effects at Invasion and Infection in Animals, in: Tez. Dokl. 4-go s’ezda po radiatsionnym issledovaniyam (radiobiologiya, radioekologiya, radiatsionnaya bezopasnost’ (Abstracts of Papers, IV Congress on Radiation Research (Radiobiology, Radioecology, Radiation Security), part 2, Moscow, 2001, p. 646.
9. Isamov N.N., Budarkov V.A. and Surgucheva L.M., Diagnostics and Specific Prophylactics of Contagious Diseases of Farm Animals in the Territories Contaminated with Radioactive Substances, Veterinarnaya patologiya, 2002, no.3, pp. 134-151.
10. Isamv N.N., The Animal Husbandry Aspects of Radiation Risks and Ecological Aftereffects of the Chernobyl Accident, Vestn. RASKhN, 1997, no. 5, pp. 64-66.
11. Isamov N.N., Animal Diseases Various Etiologies and their Modification by Ionizing Radiation, in: Mat. nauch. conf. “Nauchnoye obespecheniye veterinarnogo blagopoluchiya zhivotnovodstva” (Proc., Sci.-Pract. Congress “Science Support of Veterinary Wealth in Animal Husbandry”), Samarkand, 2001, pp. 71-72.
12. Isamov N.N. and Bulkhanov R.U., Principal of Diagnostics and Therapy at Radiation Impact against the Background of Non-Contagious Diseases, in: Mat. nauch. conf. “Nauchnoye obespecheniye veterinarnogo blagopoluchiya zhivotnovodstva v Uzbekistane” (Proc., the Sci.-Pract. Congress “Science Support of Veterinary Wealth in Animal Husbandry of Uzbekistan”), Samarkand, 1996, pp. 66-68.
13. Magomedov A.A., Pneumonia at Radiation Sickness, Veterinariya, no. 2, pp. 82-85.
14. Mandel L., Moravek F., Trebichavsky I. et al., The Germfree Animal in Radiobiology, Folia microbiologica, 1979, vol. 24, no. 1, p. 107.
15. Chukhlovin B.A., The Influence of Ionizing Radiation on Latent Salmonella Infection Occurrence, Meditsinskaya radiobiologiya, vol. 4, no. 4, p. 86.
16. Sravnitel’naya kletochnaya i vidovaya radiochuvstvitel’nost’(Comparative Radiosensitivity of Tissue Cells and Species), Bond V. and Sugakhara T., Eds., Moscow, 1974.
17. Aleksakhin R.M., Sarapultsev I.A., Spirin E.V. et al., Formation of Doses Loads in Farm Animals at the Radiation Accident in the Chernobyl Power Plant and the Influence of their Evacuation on Absorbed Doses, Dokl. RAN, 1992, vol. 323, no. 3, pp. 576-579.
18. Izrael’ Yu.A., Sokolovskii V.G., Sokolov V.E. et al., The Ecology Late Effects of Radioactive Contamination of Natural Elements in the Chernobyl Power Plant Region, Atomnaya energiya, 1988, vol. 64, no.1, pp. 28-40.
All-Russia Development and Research Institute of Agricultural Radiology and Agroecology,
|
Received August 13, 2008 |