ÓÄÊ 636/639:614.31
METHODS OF SANITARY CONTROL OF LIVESTOCK PRODUCTS. I. ENZYMOIMMUNOASSAY (ELISA) OF TETRACYCLINES
A.A. Burkin1, G.P. Kononenko1, M.A. Burkin2
The conjugates of tetracycline with albumins and gelatin, synthesized by the method of formaldehyde condensation, were used for isolation of the rabbit antibodies and optimization of the conditions of indirect solid-phase competitive enzymoimmunoassay (ELISA). The antibodies had group specificity to tetracycline, chlortetracycline, minocycline, doxycycline, metacycline and oxytetracycline. The sensitivity of used variant of ELISA during detection of tetracycline in solution was of 1ng/ml. The authors discuss the application of proposed test-system in toxicological experiments for the control of the antibiotic doses supplemented to feed rations and the determination of residual quantity in animal produce.
Key words: tetracyclines, feeds, milk, meat, immunoassay.
Tetracycline antibiotics are widely used as effective bacteriostatic substances in veterinary practice. To determine their residues in animal products, it has been developed a number of chromatographic methods (1-3), but in recent years it is increasingly being reported about the use of immunochemical approaches (4, 5).
Our purpose was the obtaining of specific immunoconjugates based on tetracycline (TC) and developing the test system for solid-phase indirect competitive enzyme-linked immunosorbent assay (ELISA) of tetracyclines.
Technique. In this work, the authors used tetracycline hydrochloride (T3258) and chlortetracycline (S4881) (“Sigma”, USA), minocycline hydrochloride, oxytetracycline, doxycycline and metacycline isolated from pharmaceutical preparations, formaldehyde and organic solvents (“Fluka”, Germany), bovine serum albumin (BSA), egg albumin (EA) and gelatin (Gel) of domestic production. Anti-species enzyme conjugate was obtained from the horseradish peroxidase (EC 1.11.1.7) and donkey antiserum to rabbit immunoglobulin according the described technique (6). ELISA was performed on high binding polystyrene microtiter plates (“Costar”, USA), optical density was measured on the photometer AKI-Ts-01 (Russia), UV spectra were recorded on the spectrophotometer Hitachi-557 (Japan).
In the reactions of protein conjugates synthesis, the authors used the solution of hydrochloride TC in dimethylformamide (10 mg/ml). The reaction products were dialyzed against three changes of 1000-fold volume of 0,5% sodium chloride solution. The equal volume of glycerol was added to the dialysate and after that stored at the temperature of – 10 °C… - 15°C.
To obtain BSA-TC (10), BSA-TC (25), BSA-TC (50) and BSA-TC (100), the aliquots of the initial solution of hydrochloride TC corresponding to its 10-, 25-, 50- and 100-fold molar excesses relative to protein and 100 ul 37% formaldehyde (1230 umol) were added to the solutions containing 5 mg BSA (0,07 umol) in 1,5 ml water. Then the reaction mixtures were stirred (48 h at 30 °C) and dialyzed.
To obtain BSA-TC (30), BSA-TC (90), BSA-TC (150), EA-TC (2), EA-TC (6), EA-TC (20), Gel-TC (4), Gel-TC (12), Gel-TC (40) and Gel-TC (120), the aliquots of the initial solution of hydrochloride TC corresponding to necessary molar excesses of the substance relative to protein and 300 ul 37% formaldehyde (3690 umol) were added to the solutions containing, respectively, 5 mg BSA (0,07 umol), 4 mg EA (0,1 umol) and 8 mg Gel (0,05 umol) in 1,5 ml water. Then the mixtures were stirred (3 h at 30 °C) and dialyzed.
Rabbits-males of gray color (weight – 2,3 kg) were immunized with a conjugate of BSA-TC (90). In the 1st injection, the animals received 100 ug immunogen in complete Freund's adjuvant subcutaneously to 10-15 points of the back, during the 2nd and subsequent injections (all performed with an interval of 1 month) - 100 micrograms in saline. In 7 days after each re-injection, the blood was collected from the ear edge vein, serum was separated, mixed with an equal volume of glycerol and stored at -10 C°... -15 C °.
Testing of each antiserum started from a non-competitive ELISA to determine antibodies’ working titer and to choose the concentration of antigen immobilized on a solid phase (0,05 or 0,15 ug/ml in 0,05 M carbonate-bicarbonate buffer, pH 9,5), which provided the intensity of analytical signal (optical density) equal to 0,8-1,2 opt. units. Then, the competitive analysis with TC solutions was performed for each combination “antibody-antigen”.
When performing ELISA, the plate wells were filled with 0,2 ml of solutions of immobilized antigens in 0,05 M carbonate-bicarbonate buffer (pH 9,5), incubated for 16 h at 4 °C and then washed 4-5 times with 0,15 M PBST (pH 7,5) (phosphate-buffered saline containing 0,01 M Na2HPO4, 0,14 M NaCl and 0,05% Tween 20). Then 0,1 ml antibody was added to PBST with 1% BSA and 0,1 ml of the same buffer (non-competitive analysis) or 0,1 ml of working TC solutions - to PBST (competitive analysis) and incubated for 1 h at room temperature, washed, and added 0,2 ml enzyme conjugate. After 1 h, the mixture was washed, introduced into the plate wells with 0,2 ml substrate solution (0,4 mg/ml o-phenylenediamine and 0,005% Í2Î2 in 0,15 M citrate-phosphate buffer, pH 5,0), and after 45 min added 50 ul 4 M sulfuric acid containing 0,1 M Na2SO3. A photometry was performed at λ = 492 nm; the average values of optical density in doubling wells were used to calculate the degree of antibody binding to immobilized antigen as the ratio A/A0 (expressed as a percentage).
To assess the analysis specificity, the working TC solutions, chlortetracycline, minocycline, oxytetracycline, doxycycline and metacycline in PBST were prepared from stock solutions in 0,1 N HCl, the concentrations of which were clarified by spectrophotometry upon values of molar extinction (e) given in the literature (7, 8). A cross-reactivity of the antibodies was defined as the ratio of concentration of TC solution providing 50% inhibition of antibodies binding to the immobilized antigen (IC50) to similar concentration of another antibiotic, and expressed as a percentage.
The test system variant optimized for sanitary control purposes was used to analyze the 8 samples of muscle tissue and internal organs of pullets (weight – 1,5 kg). During the special experiment, the pullets were previously twice (with a 1 day interval) intragastrally (by probe) administered with 150 mg TC hydrochloride dissolved in 12,5 ml water (control - the same quantity of water). In 5 h after the 2nd administration, the pullets were decapitated and the tissues (muscles, heart and liver) were sampled. The samples were homogenized and stored at -18 °C; before analyzing, the samples were dried with a thermo fan at 20-40 °C for 3-4 h.
The other investigated samples were: flour meal of animal origin (meat, fish), protein-vitamin-mineral supplements (PVMS), mixed fodders obtained from cattle farms and samples of milk from the supermarket of Moscow retail network.
Results. TC hydrochloride used as a hapten for conjugation with proteins, performed in water the absorption peaks at 275 and 358 nm, in 0,1 N HCl solution - at 269 nm (e = 20 280) and 355 (e = 15 140), which corresponded to the literature reports e = 20 054 at 270 nm, e = 13 300 at 355 nm (8). The short-wave absorption maximum of the substance most coincided the absorption peak characteristic for all protein carriers (280 nm), and, therefore, the presence of hapten in the conjugate could be detected by the absorption at λ > 340 nm.
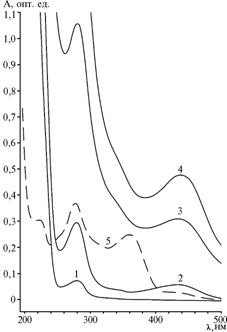
According to standard procedure of formaldehyde condensation (Mannich reaction), it is recommended to use the ratio: 25 ul 37% formaldehyde solution per 1 mg BSA and heating the reaction mixture at a temperature from 37 to 57 °C for 3-24 h (9). The obtained products of reaction with such an excess of formaldehyde and heating at 30 °C for 48 h showed the UV spectrum not diverse from BSA. Have increased the proportion of formaldehyde (60 ul per 1 mg BSA) after 3 h at the same temperature, the authors obtained the conjugates with clear spectral differences from the protein carrier. However, these products performed the absorption peak at 435 nm (TC - at 358 nm) (Fig. 1). The bathochromic shift of 77 nm indicated a profound change in the TC molecule chromophoric system, which could result from binding with proteins. The intensity of UV absorption increases regularly during the reaction with increase in hapten’s excess, and it has reached, respectively, 0,32 and 0,49 opt. units at BSA loads of 90 and 150 mol/mol. The spectra of TC conjugates with Gel demonstrated the same apparent absorption maximum at 435 nm. This mismatch in spectra of TC protein conjugates has prevented the consequent use of spectrophotometry for calculations of epitope density.
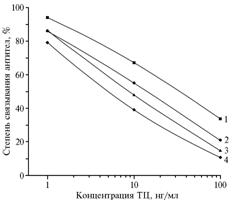
Previously, it has been revealed using radiometry that the 120-fold molar excess of TC in the formaldehyde condensation reaction provides the high degree of hapten load - up to 30-40 moles per 1 mole BSA (10), which defined the authors’ choice of the conjugate BSA –TC (90). After the 2nd injection of BSA-TC to the animals (i.e. at the first blood sampling), the specific antibodies with a titer 1:25 000 were derived, which allows to detect TC at its concentration of 10 ng/ml in the solution using the solid-phase antigen BSA-TC having a homologous protein carrier (30). Continuation of immunization procedure (up to the 4th procedure of collecting the blood) has led to an increase of test sensitivity while maintaining the high operative antibody titer (Fig. 2). Further (up to the 8th obtaining of serum), the listed indicators remained almost unchanged. Thus, despite the deep restructuring of chromophore system in the hapten molecule of immunogen, the antibodies to this showed effective binding to TC.
During the solid-phase immobilization, all four products of conjugation of BSA with TC obtained at a small quantity of formaldehyde showed negative results in a competitive ELISA. Testing of 9 conjugates heterologous for immunogen on carrier type and / or hapten load has revealed that two of them - Gel-TC (40) and Gel-TC (120) were the main conjugates providing a linear step of analytical signal (the degree of antibody binding, %) within the range 10 - 90% for TC concentrations from 1 to 100 ng/ml (Table 1). Seven others were somewhat inferior to them while retaining the immunoreactivity properties (see Table 1).
| 1. The degree of binding (%) antiserum to BSA-TC(90) with different immobilized antibodies at presence of TC | ||||
Immobilized antibody (0,05 ug/ml) |
TC, ng/ml |
|||
100 |
10 |
1 |
0,1 |
|
BSA-TC (30) |
12 |
27 |
74 |
92 |
BSA-TC(90) |
27 |
53 |
84 |
97 |
BSA-TC(150) |
49 |
67 |
93 |
99 |
Gel-TC(4) |
31 |
50 |
91 |
94 |
Gel-TC(12) |
23 |
56 |
93 |
94 |
Gel-TC(40) |
13 |
38 |
81 |
94 |
Gel-TC(120) |
15 |
23 |
70 |
89 |
EA-TC(2) |
12 |
35 |
92 |
98 |
EA-TC(6) |
24 |
50 |
82 |
94 |
EA-TC(20) |
21 |
49 |
84 |
92 |
Note: BSA, Jel, TC, EA – respectively, bovine serum albumin, gelatin, tetracycline, egg albumin. The rabbit antiserum obtained after the 9th immunization (the 8th procedure of collecting the blood). |
||||
At the competitive analysis with immobilized Gel-TC (40), it has been found a similar identification by these antibodies of tetracycline and chlortetracycline, minocycline and doxycycline, oxytetracycline and metacycline with a gradual decline in cross-reactivity levels from 100 to 20% (Table 2).
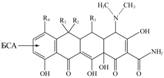
The used tetracyclines (see Table 2.) differ in the R1-4 substituent:
 ,
,
Note: ÁÑÀ - BSA
which is located in three condensed molecule cycles and may affect specificity of analysis. Apparently, the antibody recognition largely depends on nature of the substituent R1 – the distant one from sites of binding to protein, because TC and oxytetracycline showed extreme values of cross-reactivity.
| 2. Cross-reactivity of antibodies to BSA-TC(90) relative to tetracycline derivatives with different substituents | ||||||
Tetracycline derivative |
Substituent |
IC50, ng/ml |
Reactivity, % |
|||
R1 |
R2 |
R3 |
R4 |
|||
Tetracycline |
H |
OH |
CH3 |
H |
6,8 |
100 |
Chlortetracycline |
H |
OH |
CH3 |
Cl |
8,3 |
81 |
Minocycline |
H |
H |
H |
N(CH3)2 |
14,1 |
48 |
Doxycycline |
OH |
H |
CH3 |
H |
16,6 |
41 |
Metacycline |
OH |
=CH2 |
=CH2 |
H |
28,2 |
24 |
Oxytetracycline |
OH |
OH |
CH3 |
H |
33,9 |
20 |
Note: IC50 — concentration resulting in 50 % inhibition of antibodies binding to a solid-phase antigen |
||||||
The contribution of R2 was slightly smaller, since the absence of hydroxyl group in the molecule of minocycline nearly twice reduced the interaction with it. The nature of R4 (the atoms of H and Cl) in TC and chlortetracycline almost didn’t affect the recognition, possibly, owing to its proximity to the site of hapten connection with a protein. The ratio of maximum and minimum IC50 values in this series amounted to 4,7, which completely satisfies the recently proposed criteria for group specificity (11).
|
Fig. 1. UV-absorption spectra of bovine serum albumine (BSA) (1), and its conjugates BSA-TC(30) (2), BSA-TC(90) (3), BSA-TC(150) (4) and tetracycline (TC) (5). Note: abscissa – λ, nm ordinate – A, opt. units |
For the antimicrobial substances of a distinct chemical structure -
- chloramphenicol, streptomycin, penicillin, bacitracin, gentamicin and fluoroquinolones at concentrations up to 100 ug/ml, no inhibition of antibody binding to the solid phase was observed. This fact indicates the essential possibility of applying this test system for selective detection of tetracyclines in animal tissues, organs and body fluids.
The practical use of immunoassay test-systems requires their adaptation to investigated objects by selecting the optimal method of analyte extraction. In connection with ELISA, it is often performed the extraction of low-molecular substances with acetonitrile and water-acetonitrile mixtures, which, though, should imply the fact that an organic solvent presence can affect the analytical performance of a test-system, mainly – the characteristics of dependency between the detected signal and concentration of a substance, as well as sensitivity of determination.
|
Fig. 2. The degree of antibody binding to BSA-TC(90), obtained at 1st – 4th procedures of collecting the blood (1-4), with the solid-phase antigen Gel-TC(40) at presence of tetracycline. BSA, Gel, TC – respectively, bovine serum albumin, gelatin, tetracycline. Note: abscissa – Concentration of TC, ng/ml ordinate – Degree of antibody binding, % |
|
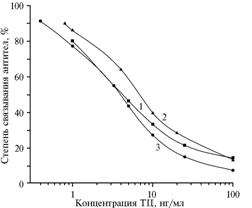
Fig. 3. ELISA calibration charts of TC with antiserum to BSA-TC(90) from the 8th blood sampling and the solid-phase antibody Gel-TC(40) (n = 10) in an PBST buffer (1), the mixture of acetonitrile with water at 10-fold dilution in PBST (2), and in milk at 3-fold dilution of PBST (3). BSA, Gel, TC – respectively, bovine serum albumin, gelatin, tetracycline. Note:
abscissa – Concentration of TC, ng/ml |
The ELISA basic principle of absolute calibration requires the accurate identity of reaction conditions in all variants including calibration solutions. To standardize the preparation of TC solutions for the analysis, the authors determined a molar extinction coefficient for the absorption maximum of substance solution in acetonitrile at 365 nm. Have measured the optical density of five TC solutions with concentration of 20 ug/ml prepared from individual portions, e amounted to 400 ± 12 900. Standard solutions were prepared by diluting of this solution with a mixture of acetonitrile and water (84:16, volume : volume) to the concentration of 1 ug/ ml.
Analytical characteristics of the developed test system were weakly dependent on acetonitrile presence: calibration charts obtained in solutions containing acetonitrile and in the buffer solution were almost identical and they provided a range of measurements TC from 1 to 100 ng/ml (Fig. 3). In intermediate conditions with variable factors (equipment, operator, regularity - daily or at intervals of 1-2 days), the relative standard deviation of antibody binding indices (n = 10) for the calibration solutions did not exceed 0,05. This fact indicates a stable operation of the test system under laboratory conditions at normal fluctuations of external factors.
3. Contents of tetracycline antibiotics in feed raw and mixed fodders upon the results of ELISA test |
||||
Test subject |
Number of samples, psc. |
Content (in TC equivalents), mg/kg |
||
total |
with binding degree |
|||
> 90 % |
< 90 % |
|||
Meat flour meal |
4 |
4 |
0 |
Not detected |
Fish flour meal |
4 |
4 |
0 |
Not detected |
PVMS |
10 |
9 |
1 |
0,1 |
Mixed fodders |
48 |
31 (from 90 to 106 %) |
17 |
0,05-0,3 (average 0,1) |
Note: PVMS – protein-vitamin-mineral supplements. |
||||
Extracts of the pullets’ tissues from the control group (the ratio of extractant and sample fresh weight of 1:1), didn’t cause any background effects after a 10-fold dilution with the buffer. Levels of antibiotic contamination of the investigated samples of muscular tissues, liver and heart amounted to, respectively, 645 ± 15, 1250 and 1260 ug/kg. These data mean that in 5 h after the 2nd introduction of TC to the bird (total dose 200 mg/kg body weight), its residual content in muscles and internal organs was approximately 1 mg/kg, i.e. 0,5% of the injected. Under the current standards, the content of residues of tetracycline antibiotics in meat products shall not exceed 10 ug/kg (12). In this regard, this becomes clear why it is recommended to stop using these preparations in therapeutic and prophylactic purposes 3 days before slaughter (13, 14).
The extracts of feeds were obtained with adding the 5-fold volume of extractant relative the mass of a sample. In this variant, the range of measurements was from 0,05 to 5 mg/kg. Most of the studied extracts were "zero": the degree of antibody binding was equal to or more than 90%, thus indicating the absence of significant impact on measurement results of co-extractives (Table 3).
Negative results obtained for the meat flour meal can be explained by the fact that antibiotics are usually added to a mixed feed. Only in 1 of 10 samples of PMVS, the presence of tetracycline antibitotics’ content equal to 0,1 mg/kg TC equivalents was proved. For the 48 tested samples of mixed fodder, 17 of those were TC-positive (the average content of antibiotics in the sample – 0,1 mg/kg). In early 1950ies, it was common in the Soviet Union to apply chlortetracycline and oxytetracycline in sub-bacteriostatic concentrations (100-1000 times smaller than therapeutic, which were 5-150 mg/kg) owing to their positive effect on growth and productivity (13, 14). The detected low content of tetracycline (0,05-0,30 mg/kg) in many tested samples of mixed fodders may reflect a persistence of such practice.
The analysis of fresh, pasteurized and sterilized cow milk has shown that its 3-fold dilution in PBST allows to determine tetracyclines in the initial product up to the content of 3 ng/ml (see Fig. 3), thereby providing the test sensitivity of 3 ug/kg. Such method of testing milk on antibiotic contamination is suitable for use in industrial conditions.
Thus, the developed ELISA test system is found to be promising for use in toxicological experiments on studying tetracyclines transmission into animal tissues and body fluids, and also for purposes of monitoring the safety of feed and animal products.
REFERENCES
1. Kennedy D.G., McCracken R.J., Cannavan A. and Hewitt S.A., Use of Liquid Chromatography-Mass Spectrometry in the Analysis of Residues of Antibiotics in Meat and Milk, Journal of Chromatography A, 1998, vol. 812, pp. 77-98.
2. Kawata S., Sato K., Nishikawa Y. and Iwama K., Liquid Chromatographic Determination of Oxytetracycline in Swine Tissues, J. of AOAC International, 1996, vol. 79, no. 6, pp. 1463-1465.
3. Ashworth R.B., Liquid Chromatographic Assay of Tetracyclines in Tissues of Food-Producing Animals, J. of the AOAC, 1985, vol. 68, no. 5, pp. 1013-1018.
4. Zhang Y., Lu S., Liu W., Zhao C. and Xi R., Preparation of Anti-Tetracycline Antibodies and Development of an Indirect Heterologous Competitive Enzyme-Linked Immunosorbent Assay to Detect Residues of Tetracycline in Milk, J. Agric. Food Chem., 2007, vol. 55, no. 2, pp. 211-218.
5. Moonsun Jeona, Jisun Kima, Ki-Jung Paengh, Sung-Woo Parke and Insook Rhee Paenga, Biotin-Avidin Mediated Competitive Enzyme-Linked Immunosorbent Assay to Detect Residues of Tetracyclines in Milk, Microchemical J., 2008, vol. 88, no. 1, pp. 26-31.
6. Nakane P.K. and Kawaoi A., Peroxidase-Labeled Antibody. A New Method of Conjugation, J. Histochem. Cytochem., 1974, vol. 22, no. 2, pp. 1084-1091.
7. Clark E.G.C., Isolation and Identification of Drugs, London, 1986.
8. Kozhybski T., Kovshyk-Gindifer Z., Kurylovich V., Antibiotiki. Proiskhozdenie, priroda i svoistva (Antibiotics. Origin, Nature and Properties), Warsaw, 1969, vol. 1.
9. Hermanson G.T., Bioconjugate Techniques, San Diego-New York-Boston-London-Sydney-Tokyo-Toronto: Academic Press, 1996, p. 785.
10. Faraj B.A. and Ali F.M., Development and Application of a Radioimmunoassay for Tetracycline, J. Pharmacol. Exp. Ther., 1981, vol. 217, no. 1, pp. 10-14.
11. Burkin M.A. and Galvidis I.A., Improved Group Determination of Tetracycline Antibiotics in Competitive Enzyme-Linked Immunosorbent Assay, Food and Agricult. Immunol., 2009, vol. 20, no. 3, pp. 245-252.
12. Gigienicheskie trebovaniya bezopasnosti i pischevoi tsennosti pischevykh produktov. Sanitarno-jepidemiologicheskie pravila i normy SanPiN 2.3.2.1078-01 (Sanitary Requirements to Safety and Nutritive Value of Food Products. The Sanitary&Hygienic Norms SanPiN 2.3.2.1078-01), Moscow, 2002.
13. Leonov N.I., Skryabin G.N. and Solntsev K.M., Antibiotiki v zhivotnovodstve (Antibiotics in Animal Husbandry), Moscow, 1962.
14. Metodicheskiye ukazaniya po primeneniyu antibiotikov v veterinarii (Methodological Instructions on the Use of Antibiotics in Veterinary), Moscow, 1974.
1 All-Russia Research and Development Institute of Veterinary Sanitation, Hygiene and Ecology, |
Ïîñòóïèëà â ðåäàêöèþ |