doi: 10.15389/agrobiology.2012.3.103eng
УДК 631.147
MANAGEMENT OF DESTRUCTION AND HUMIFICATION OF THE POST-HARVEST RESTS OF CEREAL CROPS USING MICROBIOLOGICAL PREPARATION EXTRASOL
V.B. Petrov, V.K. Chebotar'
Introduction into the soil of the wheat straw treated with microbial preparation Extrasol produced on the basis of Bacillus subtilis strain Ch-13 results in increase of activity of all main groups of microorganisms responsible for destruction and involvement of lignocellulotic compounds into the process of humus formation. In the humus structure the content of first fraction of humic acids and optic density of all complex of humic acids are mainly increasing.
Keywords: soil organic matter, post-harvest crop residues, lignocellulotic compound, humification, cereals, microbial preparation, soil microorganisms.
Humus macromolecules provide efficient viability of the biota and persistent physical, metabolic and energy properties of soil though humification is still an incompletely studied process (1-3). All its phases involve various groups of microorganisms, most important of which are fungi and actinobacteria. Humus is formed from several organic sources – aboveground and in-soil litter, root exudates of plants, waste products of soil biota, etc. (in agrocenoses – mainly from fertilizers and after-harvest crop residues) (4).
Cereal straw is a by-product with high content of cellulose, hemicellulose, and lignin in a widely varying range of C:N ratio (70-80:1), which significantly affects the type and rate of its decomposition. Cellulose-decomposing microorganisms start active functioning with a high demand for nitrogen, so the optimum C:N ratio for straw decomposition in soil is 20-30:1 (5). In Russia, utilization of straw by burning is widely plasticized in the last 20 years despite a widespread deficit in organic fertilizers. This contributes to the loss of this potential source of soil organic matter and further degradation of soil microflora had been already suppressed by misusing pesticides and agricultural chemicals. Plowing under of chopped straw in soil (another common method of utilizing organic residues of cereal crops) is also unsafe, because these substrates accumulate soil microflora (putrid saprophytes, pathogenic fungi and bacterial) that actively immobilizes soil nitrogen including that of humic substances. One more conventional technique is spraying after-harvest residues with aqueous solutions of nitric fertilizers (6), but this initiates primarily mineralization of soil organic matter to gaseous, liquid, and ballast products of metabolism. Enhancing humification by introducing of small nitrogen doses in plowed straw has no experimental and practical confirmation yet.
Today there are being developed and implied the alternative techniques for utilization of after-harvest crop residues ensuring more complete involvement of this organic matter in a biodestruction cycle by means of modern combined microbial preparations. Methodological foundations, technology and application range of such microbial preparations were established in the All-Russia Research Institute of Agricultural Microbiology (ARRIAM), in particular, preparations with a combined composition of fungi and bacteria (7-9). However, this approach becomes uneconomical in transition from small experimental sites to large-scale industrial croplands, because complex microbial compositions are often unstable and hardly adapted for use in a large-scale technology.
The purpose of this work was studying the effects of bacterial preparation Extrasol improving biodegradation and humification of after-harvest crops residues as the technique aimed at environmentally safe management of soil fertility.
Technique. A preliminary laboratory experiment was performed to evaluate cellulase activity in a sample of southern chernozem (the agricultural enterprise “Niva”, Veselovsky district of Rostov province), which changed depending on the initial level of nitrogen (20 mg carbamide solution per Petri dish), introduction of a chopped wheat straw (2 g per Petri dish), and bacterial preparations based on Bacillus subtilis strains W-13 (Extrasol) (10) and TR-6 (0,5 mg per Petri dish). Cellulase activity was determined in a Hetchinson liquid medium using filter paper (11, 12) from the difference in weight of paper fragments before and after incubation for 72 h at 20 °C (6-fold replication). A large-scale field testing of the proposed technology was conducted in August 2009, in the agricultural enterprise “Niva” on 58 ha of arable land (soil - southern arable chernozem medium loamy on underlying carbonate loess loam). The arable land (experiment) was treated with the tank mixture of 500 L water, 1 L bacterial preparation Extrasol and 25 kg active nitrogen (carbamide) per hectare. Wheat straw was chopped to pieces of 5-7 cm length and plowed under in soil immediately after spraying soil with the mixture. A control variant was 116 ha piece of the field where crop residues were burned. In July 2010, representative mixed soil samples were obtained from the arable layer (0-32 cm) of the experimental and control sites. Biodestruction hereinafter was meant as plowing under the straw treated with Extrasol against the initial dose of nitrogen, burning - as burning of after-harvest crop residues.
Standard microbiological analysis and the identification of microorganisms in the samples (11, 12) were performed using pour plate method and serial dilutions of soil suspension (10 g per 100 ml water). Parallel dilution was done to determine the count of major groups of microorganisms: Micromycetes - on Czapek's medium with lactic acid, proteolytic (ammonifying) bacteria - on MPA (meat-peptone agar), amylolytic bacteria and actinobacteria - on SAA (starch-ammonia agar), nitrogen-fixing bacteria – on Ashby medium, pedobacteria involved in humus conversion - on nitrite agar. The direction of primary transformation of organic residues in soil there was determined by measuring the amount of total carbon and nitrogen (according to Tyurin), contents of humus fractions I (plus Ia fraction of fulvic acids) and II in alkaline (0,1 N NaOH) and pyrophosphate ( 0,1M Na4P2O7 + 0,1 N NaOH) extracts (according to Kononova-Bel’chikova), optical density of humic acids with one optical filter at l=430 nm (according to Plotnikova -Ponomareva), and the content of water-soluble labile organic substances (according to Schulz- Kershenz) (13-15).
|
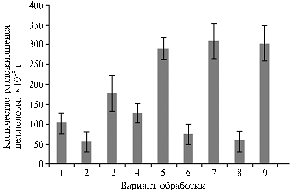
Cellulase activity patterns modified under laboratory conditions in samples of southern chernozem (Z) during decomposition of straw (2 g/Petri dish) of winter wheat (S) against the initial nitrogen supply of carbamide (C) (20 mg/Petri dish) and microbial preparations (0,5 mg/Petri dish) containing Bacillus subtilis(E — Extrasol, based on strain Ch-13; B — based on strain TR-6): 1 — Z; 2 — Z + S; 3 — Z + S + C; 4 — Z + E; 5 — Z + E + C; 6 — Z + S + E; 7 — Z + S + E + C; 8 — Z + S + B; 9 — Z + S + B + C. |
Results. In the laboratory experiment, introduction of two bacterial preparations of B. subtilis into Petri dishes with chopped straw against the initial dose of carbamide contributed to more than 3-fold increase in cellulase activity (Figure).
In the field experiment, there was observed almost complete disappearance of visually detectable fragments of straw residues treated with Extrasol during spring-summer of 2010. Significant differences in soil microbial count in the studied variants were recorded on Czapek's medium (Table 1): population growth of Micromycetes Penicillium, Trichoderma and Fusarium - the most important decomposers of plant residues in soil (their total count after Extrasol treatment more than twice exceeded that in control).
1. Count of microorganisms in soil samples (x103 CFU/g) at different techniques of after-harvest utilization of straw on southern chernozem (Enterprise “Niva”, Veselovsky district of Rostov province, 2010) |
||
Group of microorganisms |
Bio-destruction |
Burning |
Micromycetes |
19 |
9 |
Proteolytic bacteria |
11 670 |
6560 |
Nitrogen-fixing bacteria |
60 |
63 |
Amylolytic bacteria |
35 360 |
18 650 |
Actinobacteria |
1373 |
1727 |
Humus-converting bacteria |
3330 |
1312 |
Total count of bacteria |
53 812 |
31 366 |
Note: Bio-destruction – straw is plowed under in soil after treatment with Extrasol and initial dose of carbamide |
||
The count of actinobacteria and nitrogen-fixing bacteria in these variants were not significantly different. On MPA medium a population growth was shown by three ammonifying species with a predominance of fluorescent Pseudomonas. The total count of bacteria in the experiment was 1,72 times higher than in control, which indicates more intense ammonification. Using Extrasol increased the microbial count of amylolytic and humus-converting bacteria in, respectively, 1,90 and 2,54 times than in control.
Cellulase activity of soil with introduced chopped straw treated with Extrasol and carbamide (experiment), was 3,3 times higher than in the control variant with burnt crop residues. So, it was found that Extrasol activates soil microbial complex, intensifies biodestruction of crop residues and bio-conversion of nitrogen in soil. Soils of experimental and control sites had the organic matter quantity, quality, and humification degree corresponding those of arable southern chernozem (total carbon content – 2,2%, sum content of humic and fulvic acids – 0,8%, nitrogen enrichment factor of soil organic matter – 9,3-10.0).
Despite the almost similar content of total carbon, the studied samples significantly differed in the content of most mobile and active humic substances fraction I (Table 2). The increase in this fraction after Extrasol treatment can be considered as the result of intense transformation and humification of soil organic matter including crop residues.
| 2. Humus composition in soil samples at different techniques of after-harvest utilization of straw on southern chernozem (Enterprise “Niva”, Veselovsky district of Rostov province, 2010) | ||||||
Variant |
Fraction |
|||||
Humic acids |
Fulvic acids |
|||||
I |
II |
sum |
Iа + I |
II |
sum |
|
Bio-destruction |
0,11 |
0,49 |
0,60 |
0,16 |
0,05 |
0,21 |
Burning |
0,08 |
0,52 |
0,60 |
0,15 |
0,09 |
0,24 |
Note. See Table 1. Above the line – absolute value, g/100 g soil; below the line – the value relative to Ctotal, %. |
||||||
3. Optical density (Есmg/ml) in extracts of soil samples at different techniques of after-harvest utilization of straw on southern chernozem (Enterprise “Niva”, Veselovsky district of Rostov province, 2010) |
|||||
Variant |
Humic acids + fulvic acids |
Humic acids |
|||
1 |
2 |
3 |
2 |
3 |
|
Bio-destruction |
2,24 |
5,39 |
9,18 |
10,63 |
22,71 |
Burning |
2,18 |
3,74 |
8,31 |
5,43 |
21,12 |
Note. See Table 1. 1 – water extract (labile forms), 2 – extract 0,1 N NaОH (labile forms), 3 — extract 0,1 M Na4P2O7 + 0,1 N NaОH. |
|||||
Optical density of soil extracts indicates chemical maturity of humic acids. The studied soil samples notably differed in this characteristic as well (Table 3), to the greatest extent – in respect to humic acids fraction I. The observed increase in aromatization of humic substances after Extrasol treatment suggests more intense transformation of crop residues and profound humification already at its early stage. Increase in optical density of humic substances against the background of both elevated relative and absolute content of humic acids fraction I were assumed as valuable beneficial effects of the proposed technology for biodegradation of after-harvest crop residues. These substances are key important for organic fertility of soil, and the deficit of these compounds results in structural degradation of humus and total dehumification of arable chernozem in Russian South.
Introduction of microbial preparations in agrocenosis commonly leads to the following events. Firstly, the bacteria B. subtilis – a producent of various antibiotic agents – shows high competitiveness in colonization of plants, their above-ground and root litter (10). Enrichment of soil with fresh organic matter contributes to restoration and formation of new interorganismal associations, including those responsible for cellulolytic, lignin-destructive and humification processes. The presence of B. subtilis in agrocenoses complicates the structure of microbial population in annual and multiyear cycles. Already in 60 days after a single soil treatment with Extrasol, the number of bacteria morphotypes increases by 32-70%. Positive aftereffects of Extrasol on the microflora and soil fertility persisted for at least two growing seasons (16, 17).
Microbial preparations with target effects can gradually provide viability and efficient functioning of soil microorganisms that transform organic matter, which ensures necessary initial conditions and displacement of functionally useless microflora, as well as the balanced supply with energy and nutrient substrates (including the associative nitrogen fixation) in persistently functioning cycle of biodegradation and humification. Secondly, soil treatment with microbial preparations can promote the development of soil microbial communities (genetic metabolic networks) of Micromycetes, Actinobacteria and Eubacteria (while the leading role of soil fungi) that consistently decompose initial substrates and supply each other with energy and nutrients (18-20). Biodestructive activities of such networks may involve phytopathogenic microorganisms, which then become unable to attack plants (21). Spontaneous formation of effective genetic metabolic networks is low-probable, but it can be significantly enhanced by microbial preparations similar to Barkon proved as a stimulator of the formation of humifying metabolic networks of microorganisms that efficiently decompose sawdust, bark and other wood residues of coniferous trees (8, 22).
The described mechanisms are essentially consistent. After the use of both abovementioned preparations there was observed the increase in total population of microbiota and significant activation of Micromycetes, humus-converting and cellulolytic microorganisms. The most significant difference was associated with role of proteolytic and amylolytic bacteria. According to the second hypothesis, genetic metabolic networks complement the activities of fungi as an axillary element. In the experiment with Extrasol, a significant absolute increase in population was observed namely is the auxiliary groups of microorganisms, while, according to the first hypothesis, B. subtilis performs paramount functions (as well as other proteolytic and amylolytic bacteria involved in regulation of microbiocenosis, supply with nutrients and energy resources for the whole microflora involved in the process). In this view, the most promising technique for controlled transformation of organic after-harvest cereal residues is treatment with liquid microbial preparations. In the described field experiment performed in the agricultural enterprise “Niva” in 2010 the grain yield of winter wheat increased by an average of 3 kg/ha while gluten content was higher by 2,5-3,0%.
Thus, it has been successfully tested the technique for utilization of after-harvest crop residues that can be done in late summer (for winter crops) or in fall (for spring crops) by plowing straw under in soil sprayed with Extrasol with simultaneous introduction of carbamide as a source of initial nitrogen supply, which allows to convert unwanted crop residues into a valuable organic fertilizer with a high degree of humification. Extrasol is compatible with fertilizers commonly used in mix tanks. The proposed technology is cost effective, environmentally safe, and suitable for sustainable agriculture management by decomposition and humification of crop residues.
REFERENCES
1. Orlov D.S., Gumusovyekislotypochv(Humic Acids of Soils), Moscow, 1974.
2. Lykov A.D., Gumus i plodorodie pochv (Humus and Soil Fertility), Moscow, 1985.
3. Kiryushin V.I., Ekologizatsiya zemledeliya i tekhnologicheskaya politika (Ecologization of Agriculture and Technological Politics), Moscow, 2000.
4. Tate R., Organicheskoe veschestvo pochvy: biologicheskie i ekologicheskie aspekty (Soil Organic Matter: Biological and Ecological Effects), Moscow, 1991.
5. Kononova M.M., Organicheskoe veschestvo tselinnykh i osvoennykh pochv (Soil Organic Matter of Virgin and Arable Lands), Moscow, 1972, pp. 7-69.
6. Prikaz Upravleniya po ekologii i prirodopol’zovaniyu Voronezhskoi obl. ot 12.03.2007 № 132 “Ob utverzhdenii rekomendatsii po utilizatsii pozhnivkykh ostatkov i solomy” (The Order of the Department of Ecology and Environmental Management of the Voronezh Region, dated 12.03.2007 № 132 “On Approval of Recommendations for Utilization of After-Harvest Crop Residues and Straw”).
7. Sviridova O.V., Tuev N.A., Sakulina G.G. et al., The Method of Wood Decomposition, Certificate of Authorship № 1792974 dated October 8, 1992, Bul. izobr. № 5, 1993.
8. Sviridova O.V., Vorob’ev N.I. and Petrov V.B., Microbiological Destruction of Wood Waste Products and Involvement of Lignin-Containing Compounds in Agroecosystem, in Mat. nauch. konf. “Postgenomnaya era v biologii i problemy biotekhnologii” (Papers of Sci. Conf. “Post-Genomic Era in Biology and the Problems of Biotechnology”), Kazan, 2005, pp. 75-76.
9. Sviridova O.V., Vorob’ev N.I., Petrov V.B. et al., Informational Relationship between Micromycetes and Bacteria in Ecological Niches with Lignin-Cellulose Substrates, in Tez. dokl. 2-go s’ezda mikologov Rossii “Sovremennaya mikologiya v Rossii” (Abstracts of Reports, II Congress of Russian Mycologists “Modern Mycology in Russia”), Moscow, 2008, pp. 233-234.
10. Chebotar’ V.K., Zavalin A.A. and Kiprushkina E.N., Effektivnost’ primeneniya biopreparata ekstrasol (Effectiveness of Using the Bioactive Preparation Extrasol), Moscow, 2007.
11. Nekotorye novye metody kolichestvennogo ucheta pochvennykh mikroorganizmov i izuchenie ikh svoistv: Metod. rekom VNIISKhM (Some New Methods of Accounting Soil Microorganisms and Studying Their Properties: ARRIAM Guidelines), Leningrad, 1987.
12. Tepper E.Z., Shil’nikova V.K. and Pereverzeva G.I., Praktikum po mikrobiologii (Manual of Microbiology), Moscow, 1987.
13. Ponomareva V.V. and Plotnikova T.A., Metodicheskie ukazaniya po opredeleniyu soderzhaniya i sostava gumusa v pochvakh (Methodological Guidelines for the Determination of Humus Content and Composition in Soils), 1975.
14. Orlova N.E., Bakina L.G. and Orlova E.E., Metody izucheniya soderzhaniya i svoistv gumusa (Techniques for the Study of Soil Humus Content and Properties), St. Petersburg, 2007.
15. Rekomendatsii dlya issledovaniya balansa i transformatsii organicheskogo veschestva pri sel’skokhozyaistvennom ispol’zovanii i intensivnom okul’turivanii pochv (Guidelines for the Study of Soil Organic Matter Balance and Transformation during an Agricultural Exploitation of Lands and Their Intense Introduction to Cultivation), Moscow, 1984.
16. Petrov V.B., Cheborar’ V.K. and Kazakov A.E., Microbiological Preparations in Biologization of Agriculture in Russia, Dostizhaniya nauki i tekhniki APK, 2002, vol. 10, pp. 16-20.
17. Petrov V.B., Kovaleva N.M., Sviridova O.V. et al., Management of Agrocenoses’ Properties in Russian North-West Using the New Developed Microbiological Preparations, in Mat. mezregion. nauch.-prakt. konf. “Pochvennye resursy severo-zapada Rossii: ikh sostoyanie, okhrana i ratsional’noe ispol’zovanie” (Papers of Inter-Regional Sci.-Pract. Conf. “Soil Resources of Russian North-West: Current State, Preservation and Rational Use”), St. Petersburg, 2008, pp. 167-175.
18. Kolchanov N.A., Anan’ko E.A., Kolpakov F.A., Podkolodnaya O.A., Ignat’yeva E.V., Goryachkovskaya T.N. and Stepanenko I.L., Gene Networks, Molekulyarnaya biologiya, 2000, vol. 34, no. 4, pp. 533-544.
19. Kolchanov N.A., Suslov V.V. and Gunbin K.V., Biological Evolution Modeling: Genetic Regulatory Networks and Encoding of Biological Complexity, Vestnik VOGiS, 2004, vol. 2, pp. 86-99.
20. Likhoshvay V.A., Matushkin Yu.G. and Fadeev S.I., Relationship between a Genet Network Graph and Qualitative Modes of Its Functioning, Molekulyarnaya biologiya, 2001, vol. 35, no. 6, pp. 1080-1087.
21. Terekhova V.A., Mikromitsety v ekologicheskoi otsenke vodnykh i nazemnykh ekosistem (Micromycetes in Ecological Evaluation of Aquatic and Terrestrial Ecosystems), Moscow, 2007.
22. Sviridova O.V., Vorob’ev N.I., Petrov V.B. et al., The Technology for Production of Biodestructor BARKON Used to Obtain Organic Fertilizers from Coniferous Wood Waste Products, in Mat. Mezhd. kongr. “Biotekhnologiya – sostoyanie i perspektivy razvitiya” (Papers Int. Sci. Congress “Biotechnology: Current State and Prospects for Development”), Moscow, 2003, part 1, pp. 227-228.
All-Russia Research and Development Institute of Agricultural Microbiology, RAAS, St. Peterburg – Pushkin 196608, Russia, |
Received December 20, 2010
|