doi: 10.15389/agrobiology.2012.3.37eng
УДК 633.111.1:(573.6.086.83+577.21)
ANALYSIS OF VERTICAL GENE TRANSFER FROM TRANSGENIC TO NONTRANSGENIC PLANTS OF WHEAT (Triticum aestivum L.)
D.N. Miroshnichenko1, M.V. Filippov1, S.V. Dolgov1, 2
The joint Russian Collection of Agricultural Microorganisms (RCAM) was founded in 2010 at the All-Russia Research Institute for Agricultural Microbiology, Saint-Petersburg. For long-term maintenance and authorized depositing of microbial strains in RCAM the newest automated STC Tube Store at -80 °С (Liconic Instruments, Liechtenstein) is used. Liconic Instruments’ Tube Store becomes an extremely useful, simple and intuitive system for a long-term storage of microorganisms at optimum conditions without the loss of their valuable properties. This ultra low temperature store is based on the chest freezer principle. Samples are loaded into the chamber via special interface unit (-20 °С), which ensures a stable temperature and dry conditions into the storage compartment. Computer passwords used by depositors during the sample load operation ensure an authorized access to commercial strains of microorganisms. Unique «real time» online database of microorganisms has been organized on basis of the STC Tube Store Sample management software, which contains all information about the strains deposited: description, location in the store and movements of each sample. Such kind of store gives possibilities to intensify the search of new microbial cultures, keeping in mind the huge soil microbe’s biodiversity and a necessity to mobilize genetic resources of microorganisms for an agricultural production.
Keywords: transgenic wheat, crop-to-crop gene flow, transgene segregation, herbicide resistance, GFP fluorescence.
Expression of various heterologous genes in cultivated wheat varieties was shown in many scientific works published over the current decade. Eventhough genetically modified (GM) wheat hasn’t been introduced in agricultural practice yet, it is quite predictable in coming years as a logical continuation of mote than 500 field trials of transgenic wheat already performed in different parts of the world including Europe, USA, Canada, Japan, Australia, China, Mexico, and Russia since 1996. In Russia wheat is the main food crop, and all aspects of its genetic modification are very significant.
A flow of transgenes from GM plants to non-transgenic ones, or so-called vertical gene transfer, is one of possible problems in a large scale growing of GM crops. Manifestations of its aftereffects can be expected in regions with overlapped areas and synchronized flowering periods of transgenic crops and conventional varieties, as well as in neighboring wild or weedy relative species.
Wheat is annual self-pollinating crop, which minimizes gene flow through pollen. Soft wheat is known as a crop having the minimum occurrence of cross-pollination – 0,5-10,0% depending on a variety. This fact is conditioned by biological peculiarities of the species: its pollen grains are heavier and smaller in number than in other cereals (2), so about 90% wheat pollen settles within 3 m (3, 4). In some cases, it may be carried by the wind to a distance of 50-60 meters (2, 3). Viability of wheat pollen is believed as relatively low, being maintained for an average of 20-30 min (2, 5), although it can vary from a few minutes to 2-3 hours in different varieties (6). Another important biological feature of wheat is the frequency of anther disclosure during a flowering reaching 61-93% in different cultivars (7).
The low frequency of cross-pollination in wheat allowed the European Environment Agency (EEA) and the European Science Foundation (ESF) to consider wheat as the crop a very low risk of vertical transfer of transgenes into the genome of wild relative species (8). Despite this, cultivation of wheat can’t exclude the possibility of vertical gene flow from genetically modified to non-modified varieties with pollen and development of grains-hybrids for heterologous genes, which than can affect seed purity and grain quality.
Vertical gene transfer through pollen is being studied in cereals since the end of the past century. The most famous works focused on this issue were conducted on maize (9), sorghum (10), rice (11), and barley (12). In wheat, gene flow with pollen was studied mainly in non-transgenic varieties (13, 14). The only available report on vertical transfer of transgenes in wheat (15) shows obtaining of hybrid transgenic F1 by cross-pollination of jointly grown non- transgenic and GM plants-donors of genes bar and gus, but the frequency of gene flow wasn’t accounted and the analysis of influencing factors wasn’t performed owing to insufficient sample size.
In this research the authors studied vertical gene transfer from transgenic to non-transgenic soft wheat plants and the frequency of transgene transfer depending on directions of prevailing winds, remoteness of non-transgenic recipients relative to transgenic pollen donors along with transgene inheritance patterns in a following seed generation.
Technique. The experiments were conducted for 2 years (2004 and 2005). The object of study – non-transgenic plants of spring soft wheat cv Andros and its transgenic forms obtained in the artificial climate station “Biotron” (Puschino branch of the Institute of Bioorganic Chemistry, RAS, Moscow province) through ballistic transformation with psGFP-BAR vector (16). Transgenic object of study was homozygous seed progeny T3 selected after the reproduction of a primary transgenic plant A-20 carrying bar gene for acetyl phosphotranspherase including ubil intron of maize and controlled by ubil promoter of maize (17), and gfp gene for green fluorescent protein (GFP) with act1 intron of rice controlled by act1 promoter of rice (18). Experimental plot was laid in a quarantine nursery garden of the All-Russia Research and Development Institute of Fruit Crop Selection (Orel). The test plot was located at least 10 km distant from commercial wheat croplands.
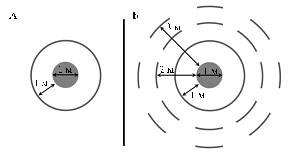
In both years of the study, transgenic wheat seeds were sown within the circle with a diameter of 1 m at sowing rate 500 pcs/m2. In the first year (2004), non-transgenic seeds were sown in 2 rows with interrow spacing 10 cm at a distance of 1 m from the outer perimeter of the circle with transgenic wheat; in the 2nd year (2005) - at a distance of 1, 2 and 3 m from the outer perimeter. The first round of non-transgenic recipients was continuous, the 2nd and 3rd rounds (2nd year of study) were sown as eight sectors (1 m) corresponding to the cardinal. Before harvesting ears, the first continuous circle was also divided into eight equal sectors according to the cardinal.
To assess vertical gene flow of transgenes, F1 seeds from non-transgenic plants were sown in winter greenhouses on shelves of a multilevel rack filled with a mixture of peat and sand (3:1). Wheat seedlings of 9-11 cm height were treated with 1% solution of Basta herbicide (“Bayer Crop-Science”, Germany) 1-fold in the evening hours according to the manufacturer's instruction. In a week after the treatment the survived plants were dug out, transplanted into individual pots where they continue to grow.. Gfp gene activity in pollen from F1 plants was revealed by fluorescence investigation using a light microscope ICM 405 (“Opton”, Germany) with filters 450-490 nm (the wavelength of excitation) and 515-530 nm (the wavelength of GFP protein extinction).
Genomic DNA of F1 herbicide-resistant plants was extracted as described (19) using 2 x CTAB-buffer; total matrix RNA (mRNA) – according to a proposed procedure (20). The presence of inserts gfp and bar genes in the genome of F1 plants was detected by PCR, the presence of analogous fragments in cDNA synthesized from mRNA - by PCR with reverse transcription (RT-PCR). The following pairs of primers were used: for bar gene – bar for 5'-TGC ACC ATC GTC AAC CAC TA-3', bar rev 5'-ACA GCG ACC ACG CTC TTG AA-3' (size of expected fragment — 310 bp); for gfp gene — sgfp for 5'-GCG ACG TAA ACG GCC ACA AG-3', sgfp rev 5'-CCA GCA GGA CCA TGT GTG ATC G-3' (size of expected fragment — 600 bp). Amplification was performed under the regime: start at 95 °С, 5 min; denaturation at 94 °С, 45 s; elongation at 72 °С, 45 s; annealing temperature — 60 °С, 40 s (30 cycles). Amplification was performed on MasterCycler Gradient device (“Eppendorf”, Germany). Amplification products were separated in electrophoresis chamber (“Hoeffer”, USA) with 1,2% agarose gel in 0,5 x TAE-buffer supplemented with ethidium bromide using M23 marker (“SibEnzyme”, Russia).
Inheritance of transgenic trait in the seed progeny was evaluated from GFP fluorescence in young wheat germs F2 in vitro on the 2nd-3rd day after the initiation of germination.
Statistical analysis was performed using Pearson's chi-squared test (21).
Results. Location of transgenic and non-transgenic plants in test plots is shown in Figure 1.
In the 1st and 2nd years of the study, there was observed synchronous development of transgenic and non-transgenic plants after germination (tillering, booting, earing and flowering). In the 1st year, flowering occurred from June 26 to July 13, in the 2nd – from June 25 to July 9.
During flowering of wheat prevailing wind directions were similar (west, north-west and north), but the windspeed in the 2nd year was lower with a shorter total wind run (Table 1).
|
Fig. 1. Schematic layout of test plots for the study of vertical gene transfer from transgenic to non-transgenic wheat plants in 2004 (A) and 2005 (B) (quarantine nursery of the All-Russia Research and Development Institute of Fruit Crop Selection, Orel). |
In the 1st year, the total number of seeds F1 harvested from non-transgenic wheat grown at 1m distance from transgenic pollen donors amounted to 62 221 psc., in the 2nd – over 125 thousands. Climatic conditions of the 2nd year were less favorable (droughtier), which resulted in smaller yield of seeds from non-transgenic plants compared with the previous year (Table 2), while the average weight of 1000 seeds reduced by 14%.
1. Duration of flowering (DF) in transgenic and non-transgenic wheat plants cv Andros and weather conditions in test plots at this period in different years of study (600-2100, quarantine nursery of the All-Russia Research and Development Institute of Fruit Crop Selection, Orel). |
||||||||||||
DF, days |
Average air temperature, °С |
Relative air humidity, % |
Total wind run, m |
Participation in prevailing wind direction, % |
||||||||
N |
N-E |
E |
S-E |
S |
S-W |
W |
N-W |
windless |
||||
2004 |
||||||||||||
18 |
18,1 |
74,4 |
3877 |
18,0 |
2,3 |
1,6 |
4,7 |
5,5 |
10,2 |
25,8 |
28,1 |
3,9 |
2005 |
||||||||||||
16 |
18,5 |
67,3 |
2959 |
27,5 |
4,2 |
6,3 |
2,1 |
2,1 |
8,4 |
24,2 |
17,9 |
7,4 |
Note. Wind direction: N – north, N-E – north-east, E – east, S-E – south-east, S – south, S-W – south-west, W – west, N-W – north-west. |
||||||||||||
2. Occurrence of seeds F1 carrying bar and gfp genes in non-transgenic wheat plants cv Andros depending on their remoteness from transgenic plants (quarantine nursery of the All-Russia Research and Development Institute of Fruit Crop Selection, Orel). |
||||||||||||
Plot |
Number of seeds F1 harvested from non-transgenic plants, pcs. |
Number of seedlings F1 resistant to herbicide (bar+/gfp+), pcs. |
Frequency of vertical gene transfer (gene flow), % |
|||||||||
2004 |
2005 |
2004 |
2005 |
2004 |
2005 |
|||||||
1 m |
1 m |
2 m |
3 m |
1 m |
1 m |
2 m |
3 m |
1 m |
1 m |
2 m |
3 m |
|
N |
8468 |
5539 |
3902 |
3056 |
8 |
5 |
0 |
0 |
0,094 |
0,090 |
0 |
|
N-E |
8020 |
6312 |
5967 |
4930 |
49 |
13 |
4 |
1 |
0,611 |
0,206 |
0,067 |
0,020 |
E |
6660 |
5604 |
3382 |
5839 |
53 |
13 |
1 |
0 |
0,796 |
0,232 |
0,030 |
0 |
S-E |
7404 |
5571 |
5945 |
5311 |
59 |
17 |
1 |
0 |
0,797 |
0,305 |
0,017 |
0 |
S |
7980 |
5161 |
4398 |
5565 |
45 |
11 |
1 |
0 |
0,564 |
0,213 |
0,023 |
0 |
S-W |
9071 |
6613 |
4748 |
7860 |
24 |
1 |
2 |
0 |
0,265 |
0,015 |
0,042 |
0 |
W |
8400 |
6352 |
4712 |
3500 |
18 |
4 |
4 |
0 |
0,214 |
0,063 |
0,085 |
0 |
N-W |
8446 |
6617 |
3837 |
4398 |
4 |
0 |
0 |
0 |
0,047 |
0 |
0 |
0 |
Total |
62221 |
47669 |
36891 |
40459 |
259 |
64 |
13 |
1 |
0,416 |
0,134 |
0,035 |
0,002 |
Note. Denotations of test plots with prevailing wind direction (accordingly, north, north-east, east, south-east, south, south-west, west, north-west) - see Table 1. |
||||||||||||
In the 1st year, when all harvested seeds F1 were sown in winter greenhouses and 10-days old sprouts were treated with herbicide, it caused death of more than 99,5% seedlings. At the same time, some plants survived the treatment with no symptoms of damage, continued a normal growth and developed leaves. The share of herbicide-resistant plants F1 obtained from non-transgenic individuals grown at 1 m distance from transgenic plants (total number of survived plants – 259) varied significantly depending on location of recipients relative to the central circle (Table 2). In the northern and north-western sectors, there were recorded 1-2 hybrid seeds which then gave herbicide-resistant plants, while in the eastern and south-eastern – 8 psc. per 1000 seeds.
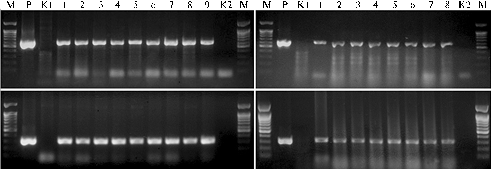
Testing the expression of GFP gene, the synthesis of this marker protein was detected in all 259 herbicide-resistant plants F1 by investigation of pollen (absence of chlorophyll facilitates visualization). DNA PCR (Fig. 2, A) ultimately confirmed the presence of transgene in the genomic sequences of all tested hybrids F1, because all the samples manifested amplification of fragments with expected length - 310 bp (bar) and 600 bp (gfp). RT-PCR of bar and gfp genes expression using total plant RNA (Fig. 2, B) also proved the presence of transgene insertion showing a successful transcription of mRNA providing the synthesis of acetyl phosphotransferase responsible for herbicide resistance, as well as marker fluorescent protein GFP. As it was found in earlier authors’ researches (16), bar and gfp in transgenic pollen donors are inherited as linked genes, because they both were initially provided by the same vector construct psGFP-BAR used to design the transgenic line A-20.
A B |
|
Fig. 2. Typical electrophoregrams of amplicons obtained in soft wheat cv Andros total DNA by PCR (A) and total cDNA by RT-PCR (B) revealing the presence of genes gfp (top row) and bar (bottom row) in the genome of herbicide-resistant plants F1 detected in a seed progeny of non-transgenic plants grown in 1 m distance from transgenic pollen donors: М — molecular weight size marker (1500, 1000, 900, 800, 700, 600, 500, 400, 300, 200 and 100 bp); P — positive control (DNA of plasmid psGFP-BAR used for design of the transgenic line); K2 — reaction mixture not containing DNA; 1-9 — hybrid plants F1 (respectively, 9/2, 1/10, 4/7, 5/15, 4/4, 4/9, 5/12, 4/13 and 17/1); size of expected fragments for gene bar — 310 bp, gfp — 600 bp. |
So, in the 1st year, molecular biological analysis revealed that all herbicide-resistant plants F1 were carriers of bar and gfp genes insertions in the genome, which was obtained during a field experiment in a test plot trhough natural cross-pollination of transgenic and non-transgenic wheat plants.
In the 2nd year of the study, non-transgenic recipient plants were sown in additional remote rows surrounding transgenic plants in a distance of 2 m and 3 m. After the herbicide treatment of sprouts F1 there remained 78 survivors with no symptoms of damage. Survival ratio of plants F1 varied significantly depending on remoteness of recepients from transgenic pollen donors. Non-transgenic recipients grown in 3 m and 2 m distance from transgenic plants developed herbicide-resistant F1 from, respectively, 1 and 13 seeds (Table 2). In 2 m distance, hybrid F1 plants occurred with frequency varying from 0 (north and north-eastern regions) to 4 pcs. (north-eastern and western regions). In the distance of 3 m the only herbicide-resistant F1 plant was recorded in north-eastern section. In non-transgenic wheat grown in 1 m distance from transgenic pollen donors the herbicide-resistant F1 progeny was represented by 64 seeds, and the rate of vertical gene transfer varied over the regions: in the south-east – 3 per 1000 seeds, and none in the north-west (Table. 2).
Fluorometric investigation of pollen from all 78 F1 plants confirmed the transfer of linked determinants for fluorescence and herbicide resistance. Molecular biological analysis (PCR and RT-PCR) showed the results similar to findings of physiological and biochemical analyses. PCR revealed in the genome of all analyzed F1 hybrid plants the presence of gfp and bar gene sequences, and their expression at mRNA level. Thus, plants F1 selected with the use of herbicide treatment were true transgenic for bar and gfp genes transferred with transgenic pollen at natural cross-pollination.
To confirm the pollen origin of herbicide resistance in transgenic F1 and disprove the possibility of accidental entry of seeds from transgenic pollen donors, the authors analyzed inheritance patterns of heterogeneous genes at further seed reproduction. In the case of a seed progeny from non-transgenic plants pollinated with pollen of homozygous transgenic line A-20, these F1 plants must be heterozygotes for the transferred genes bar and gfp. Further self-pollination of such F1 plants must provide the inheritance with a mono-locus insertion pattern, since bar and gfp genes have promotors act1 and ubi1 of rice and maize determining dominant traits. Should F1 plants be obtained from accidentally entered seeds from homozygous transgenic plants, no split occurs and fluorescence must be observed in all these descendants.
Inheritance pattern of gfp gene was studied in 14 691 grains F2 (Table 3). Although the expression level of gfp varied in these transgenic descendants, the progeny of all 337 F1 hybrid plants manifested a stable inheritance of the heterologous gene. Statistical analysis with c2-test showed that most of these plants obtained in both years of study had a mono-locus inheritance of the trait 3:1 (Table 3), even despite fluctuations in the actual ratio of transgenic and non-transgenic descendants F2 derived from individual plants F1 from 1:1 to 11:1. It should be noted that most of such “atypical” splits were observed in plants that developed a small number of seeds (10-20 pcs.).
3. Analysis of inheritance of expressing gfp gene in the seed progeny of hybrid plants F1 obtained through a vertical gene transfer from transgenic to non-transgenic wheat plants cv Andros depending on their remoteness from transgenic plants (artificial climate station “Biotron”, the branch of RAS Science and Research Development Institute of Bioorganic Chemistry, Puschino). |
|||||
Year |
A |
B |
C |
D |
E |
2004 |
1 |
259 |
9970 |
239 |
92,3 |
2005: |
|
|
|
|
|
total |
|
78 |
4721 |
68 |
87,2 |
including |
1 |
64 |
3842 |
57 |
89,1 |
Note. A – distance between transgenic and non-transgenic plants, m; B – number of bar+/gfp+ plants F1, psc.; C — number of embryos bar+/gfp+ plants F1 tested for expression of GFP, psc.; D — number of bar+/gfp+ plants F1 manifesting a split for gfp 3:1 according to c2, psc.; E — proportion of embryos manifesting a split 3:1, %. |
|||||
At the same time, there weren’t found any F1 plants with GFP fluorescence inherited as a homozygous trait. All herbicide-resistant F1 plants showed heterozygous inheritance of the transgenic determinant, which confirmed the fact of vertical gene transfer through a natural pollination with transgenic pollen.
A B |
|
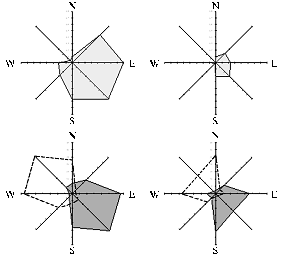
Fig. 3. Frequency of vertical transfer of transgenes bar and gfp(top row) depending on prevailing wind direction (bottom row) on non-transgenic wheat plants cv Andros grown at 1 m distance from transgenic plants in 2004 (A) and 2005 (B). |
The frequency of vertical gene flow of transgenes bar and gfp in test plots varied in different years, which could be caused by the influence of different environmental factors during the active flowering of wheat. Thus, for recipient plants located in 1 m from the pollen donor, this value averaged to, resp., 0,416 and 0,134% in 2004 and in 2005 (Table 3). Probably, the excess recorded in the 1st year of study was the result of climatic peculiarities of flowering season in the 2nd year: its duration was generally shorter, air temperature during ejection of anthers was higher against a lower humidity, pollen flow was smaller due to the reduce in total wind run (Table 1.). The value of gene flow frequency was also influenced by generally smaller yields in 2005 (by 23%) due to unfavorable water regime (insufficient precipitation).
8-Sectoral separation of the circular test area according to the cardinal was arranged in order to reveal a correlation between the occurrence of transgenic hybrid seeds and prevailing wind directions during a flowering. The direction of predominate winds in both years was similar (west, north-west and north) (Figure 3). Most of transgenic hybrid F1 seeds were recorded in four sectors located correspondingly and in the south-east. In the 1st year, in the north-eastern, eastern, south-western and southern regions (relative to the center of the circle) there were detected 206 transgenic hybrid seeds (81% of the total), in the 2nd – 45 seeds (84% of the total). In both years, the highest frequency of gene transfer was recorded in the region subject to north-western wind: in 2004 and 2005 it amounted to 0,797 and 0,305%, resp. (or 59 and 17 herbicide-resistant plants).
A distinct distribution of hybrid seeds was observed in non-transgenic plants grown in southern, south-eastern, eastern and north-eastern sectors. The share of hybrid seeds obtained in these regions was only 19% (2004) and 16% (2005) of their total number. In both years the minimum frequency of transgene transfer was recorded in the north-eastern area. These data suggest that total amount of transgenic pollen carried by the wind varied from 4 to 5 times depending on wind direction, which caused the observed fluctuations.
Increasing the distance between non-transgenic plants and transgenic pollen donors caused an obvious reduce in probability of transgene flow with pollen. In 1m distance the frequency of transgene transfer averaged 0,134%, in 2 m distance it declined almost 4 times (0,035%), and in 3 m - 66 times (0,002%). These data were generally consistent with available reports about the frequency of cross-pollination in some wheat varieties under different conditions (1, 3, 5, 6, 13, 14).
Conclusions of this research are based on interpretation of experimental data obtained in order to find out how great is the threat of transgenic pollen transfer in natural conditions. These data allow to consider different influencing factors (remoteness of recipient plants, prevailing wind direction, seasonal and climatic features) important for determining the conditions minimizing or preventing the transfer of transgenic determinants with pollen. This will help to avoid contamination of non-transgenic grain with transgenic admixture (and vice versa), as well as provide genetic integrity of wheat varieties cultivated in croplands not excluding a possible “contact” between conventional and genetically modified varieties.
So, for the first time in Russia was performed a field experiment on wheat showing the possibility of vertical transfer of transgenes at neighboring growing of forms with similar phenology. The obtained data suggest that safe isolation of transgenic crops from non-transgenic ones requires an in-depth research on a larger sample size, remoteness of plants to a greater distance, recording the volume of transgenic pollen carried by the wind depending on plot area, etc.
REFERENCES
1. Lawrie R.G., Matus-Cadriz M.A. and Hucl P., Estimating Outcrossing Rates in Spring Wheat Cultivars Using the Contact Method, Crop Sci., 2006, vol. 4, pp. 247-249.
2. D’Souza V.L., Investigations Concerning the Suitability of Wheat as a Pollen-Donor for Cross Pollination by Wind as Compared to Rye, Triticale and Secalotricum, Zeitschrift fur Pflanzenzuchtung, 1970, vol. 63, pp. 246-269.
3. Khan M.N., Heyne E.G. and Arp A.L., Pollen Distribution and the Seed Set on Triticum aestivum L, Crop Sci., 1973, vol. 13, pp. 223-226.
4. Virmani S.S. and Edwards I.B., Current Status and Prospects for Breeding Hybrid Rice and Wheat, Adv. Agron., 1983, vol. 36, pp. 145-214.
5. De Vries A.P., Flowering Biology of Wheat Particularly in View of Hybrid Seed Production: a Review, Euphytica, 1971, vol. 20, pp. 152-170.
6. Hegde S.G. and Waines J.G., Hybridization and Introgression between Bread Wheat and Wild and Weedy Relatives in North America, Crop Sci., 2004, vol. 44, pp. 1145-1155.
7. Rajki E., Effect on Pollination of the Amount of Pollen on the Surface of the Stigm, Növénytermelés, 1962, vol. 11, pp. 35-44.
8. Eastham K. and Sweet J., Genetically Modified Organisms (GMOs): The Significance of Gene Flow through Pollen Transfer, Environmental Issue (Report ¹ 28), Copenhagen: European Environment Agency, 2002, p. 75.
9. Ma B.L., Subedi K.D. and Reid L.M., Extent of Cross-Fertilization in Maize by Pollen from Neighboring Transgenic Hybrids, Crop Sci., 2004, vol. 44, pp. 1273-1282.
10. Schmidt M. and Bothma G., Risk Assessment for Transgenic Sorghum in Africa: Crop-to-Crop Gene Flow in Sorghum bicolor (L.) Moench, Crop Sci., 2006, vol. 46, pp. 790-798.
11. Messeguer J., Fogher C., Guiderdoni E., Marfa V., Catala M.M., Baldi G. and Mele E., Field Assessments of Gene Flow from Transgenic to Cultivated Rice (Oryza sativa L.) Using a Herbicide Resistance Gene as Tracer Marker, Theor. Appl. Genet., 2001, vol. 103, pp. 1151-1159.
12. Ritala A., Nuutila A.M., Aikasalo R., Kauppinen V. and Tammisola J., Measuring Gene Flow in the Cultivation of Transgenic Barley, Crop Sci., 2002, vol. 42, pp. 278-285.
13. Matus-Cadiz M.A., Hucl P., Horak M.J. and Blomquist L.K., Gene Flow in Wheat at the Field Scale, Crop Sci., 2004, vol. 44, pp. 718-727.
14. Hanson B.D., Mallory-Smith C.A., Shafii B., Thill D.C. and Zemetra R.S., Pollen-Mediated Gene Flow from Blue Aleurone Wheat to Other Wheat Cultivars, Crop Sci., 2005, vol. 45, pp. 1610-1617.
15. Gatford K.T., Basri Z., Edlington J., Lloyd J., Qureshi J.A., Brettell R. and Fincher G.B., Gene Flow from Transgenic Wheat and Barley under Field Conditions, Euphytica, 2006, vol. 151, pp. 383-391.
16. Filippov M.V., Miroshnichenko D.N., Vernikovskaya D.I. and Dolgov S.V., Optimization of Ballistic Transformation of the Soft Wheat Genome, S.-kh. biol., 2006, no. 1, pp. 67-73.
17. Christensen A.H., Sharrock R.A. and Quail P.H., Maize Poly-Ubiquitin Genes: Genes, Structure, Thermal Perturbation of Expression and Transcript Splicing, and Promoter Activity Following Transfer to Protoplast by Electroporation, Plant Mol. Biol., 1992, vol. 18, pp. 675-689.
18. McElroy D., Zhang W., Cao J. and Wu R., Isolation of an Efficient Actin Promoter for Use in Rice Transformation, Plant Cell, 1990, no. 2, pp. 163-171.
19. Rogers S. and Bendich A., Extraction of Total Cellular DNA from Plants, Algae and Fungi, in Plant Molecular Biology Manual, Gelvin S. and Schiperoort R., Eds, Dordrecht, Boston, London: Kluwer Academic Publishers, 1995, section 7-1.
20. Gehrig H., Winter K., Cushman J., Borland A. and Taybi T., An Improved RNA Isolation Method for Succulent Plant Species Rich in Polyphenols and Polysaccharides, Plant Mol. Biol. Rep., 2000, vol. 18, pp. 369-376.
21. Lakin S.F., Biometriya: uch. pos. dlya biol. vuzov (Biometrics: Manual for Biological High School), Moscow, 1990.
1Puschino Branch of M.M.Shemyakin–Yu.A.Ovchinnikov Institute of Bioorganic Chemistry, RAS, |
Received April 24, 2011
|