doi: 10.15389/agrobiology.2012.3.75eng
УДК 633.11.«324»:575.224.232.3
INFLUENCE OF EXOGENOUS HYDROGEN PEROXIDE ON ANTIOXIDANT SYSTEM OF WHEAT CHLOROPLAST
N.G. Gambarova1, V.K. Gins2
The effect of different concentrations (1 mM and 10 mM) of exogenous Н2О2 on activity of superoxide dismutase (SOD), glutathione reductase (GR) and glutathione transferase (GT) was studed using isolated chloroplasts of 14-day seedlings of heat stable wheat of the Sharg variety. It was shown, that under conditions, which exclude synthesis of SOD and GT, their activities increase and this causes additional protection of plastids at the early stage of the extreme factors' action. The rise of Н2О2 dose results in the increase of GR stability.
Keywords: chloroplast, hydrogen peroxide, superoxide dismutase, glutathione reductase, glutathione-S-transferase.
Antioxidant (AO) systems suppress the production of reactive oxygen species (ROS) and prevent accumulation of macromolecules in cells influenced by oxidative modification under various adverse impacts (1). Adaptive increase of antioxidant activity can be the indicator of organism’s stress tolerance level. Enhanced functioning of AO system with no changes in oxidative processes reflects the development of cellular oxidative stress and a violation in pro-oxidant-antioxidant homeostasis (2).
Antioxidant response to stressful impact has a number of non-specific features. Thus, stress reaction in plants is accompanied by the increase in activity of antioxidant enzymes and a pool of low-molecular-weight antioxidants along with their oxidation. The similar antioxidant response was observed in plants exposed to extreme temperatures (3), salinity (4), and drought (5). However, it is possible that exogenous Н2О2 shows a distinct mechanism of action on AO content and activity.
Today, hydrogen peroxide is considered not only as a stress factor in model experiments (6), but also as a signal messenger (7), and a regulator of defensive responses (8). This substance was shown as inducer of resistance to oxidative stress in tobacco plants by increasing the activity of AO enzymes and / or their content (9).
The purpose of this work was studying the effect of exogenously administered hydrogen peroxide on the antioxidant system of isolated chloroplasts of wheat.
Technique. The object of study – 14-day-old seedlings of wheat (Triticum aestivium L.) a heat-resistant variety Sharg. Chloroplasts were isolated from the middle part of leaves (10). 0,2 ml chloroplasts treated with 1 mM or 10 mM Н2О2 was placed in the reaction medium to determine enzyme activity of superoxide dismutase (SOD, EC 1.15.1.1) (12), glutathione reductase (GR, EC 1.6.4.2) (12) and glutathione-S-transferase (GT, EC 2.5.1.18) (13) after 5, 10, 15 , 30 and 60 minutes. Protein content in the suspension of chloroplasts was determined according to Lowry (14), the content of reduced (GSH) and oxidized (GSSG) glutathione – using the method of N.V. Shalygo et al. (15). Two experiments were performed in three replicates (n = 6). The presented data show mean values and their standard errors (16).
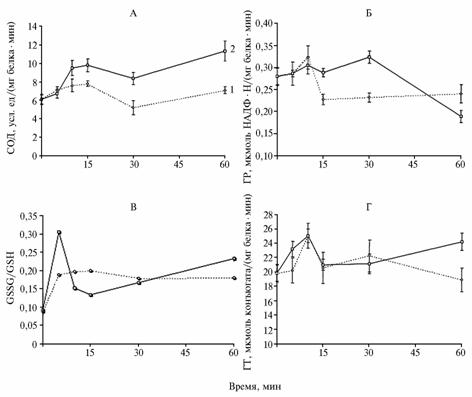
Results. Introduction to the chloroplast suspension of 1 and 10 mM hydrogen peroxide caused in both samples similar patterns of changes in SOD activity, which suggests the existence of a direct dose-response relationship (Figure, A). Н2О2 at a dose of 1 mM provided 120 % increase of SOD activity relative to a baseline with a subsequent decline, while 10 mM Н2О2 caused its 150-160 % increase remaining in this range until the end of experiment (175 % by the 60th minute).
|
Enzyme activity patterns of superoxide dismutase (SOD, A), glutathione reductase (GR, B), glutathione-S-transferase (GT, D), and GSSG/GSH ratio (oxidized glutathione/ reduced glutathione) in chloroplasts of 14-days-old seedlings of wheat Triticum aestivium L. heat-resistant variety Sharg under exogenous treatment with hydrogen peroxide (1 and 2 – resp., 1mM and 10 mM Н2О2). |
These data demonstrate the ability of Н2О2 to regulate SOD activity in chloroplasts regardless of its synthesis de novo. Enhanced activity of the enzyme induced by H2O2 micromolar concentrations was previously described in SOD of animal erythrocytes, while its millimolar doses induced rapid degradation of the enzyme (17). As a possible mechanism of Н2О2 action there was assumed its interaction with active site of the enzyme.
GR activity in chloroplasts treated with 1 mM Н2О2 in 15 min reduced by 80% of the initial and remained unchanged till the end of the experiment (Fig., B). In this case, a further growth of GSSG/GSH ratio contributed to a drop of GR activity. Under the higher dose of hydrogen peroxide (10 mM) the enzyme showed significant resistance to the stressor: its activity declined up to 70% of the initial only by the 60th minute. GR is the enzyme responsible for maintenance of intracellular glutathione pool in the reduced state; it is involved in GSH restoration from GSSG through NADFH-dependent reaction. The observed reduce in GR activity after a different period of time indicates a lack of substrate for the efficient formation of GSH in the presence of stress factor. It is known that defensive responses occur without the destruction of GSH, but it is converted to the oxidized form, which leads to the increase of GSSG/GSH ratio in stressful conditions.
The observed reduce in GR activity, apparently, resulted from a deficit of reducing equivalents in chloroplasts during the oxidative stress (18), and high resistance to 10 mM H2O2 could be the effect of adaptive responses of regulatory mechanisms providing functioning of AO systems in extreme conditions. In particular, the maintenance of increased GR activity in chloroplasts treated with10 mM Н2О2 for 30 minutes was accompanied by the growth of GSSG/GSH ratio (Fig., B).
Hydrogen peroxide in both experimental doses contributed to the rise of GT activity (up to 125% of baseline) in 10 minutes after exogenous administration of H2O2 to the suspension (Fig., D), followed by a decline of this trend. In the variant of 10 mM Н2О2, a second peak of GT activity was detected in 60 minutes. Earlier, a similar fact was observed in chloroplasts subject to heat shock (19). Despite some differences (under heat shock, a maximum GT activity occurred after 5 min), a similar pattern of response suggests the presence of a common mechanism that regulates the activity of glutathione-S-transferase under these stressors. Possibly, heat shock effect on the enzyme is mediated by elevation of ROS (including Н2О2) contents in the chloroplasts, which is quite probable knowing the ability of GT to allosteric regulation. In this case, Н2О2 itself can be considered as GT inducer.
Thus, exogenous administration of hydrogen peroxide at different doses to a suspension of wheat chloroplasts revealed the features of their antioxidant defense system, which showed considerable sensitivity and lability, and the adaptive response already in 5 min after the first contact with this stressor. In the studied model system of chloroplasts a direct response to the stressor was manifested as activation of superoxide dismutase and glutathione-S-transferase. SOD of wheat chloroplasts showed enzymatic activity under conditions precluding the synthesis of additional amounts of the enzyme. Patterns of activity of GR – a key enzyme in ascorbate-glutathione cycle – suggest its participation in regulatory responses associated with the development of non-specific response to oxidative stress. The importance of glutathione for maintenance of chloroplasts’ functional activity under extreme conditions results from both own antioxidant properties and its role in functioning of enzyme systems. Under the action of Н2О2 there increases utilization of GT in reactions with its participation, while a possibility of its recycling by GR is limited. Apparently, the increased content of Н2О2 10 mM activates lipid peroxidation and simultaneously stimulates enzyme activity of GT involved in reactions aimed at protection of lipids. Findings of this work suggest using the response of antioxidant system as a marker of oxidative stress.
REFERENCES
1. Polesskaya O.G., Rastitel’naya kletka i aktivnye formy kisloroda (Plant Cell and Reactive Oxygen Species), Moscow, 2007.
2. Mittler R., Oxidative Stress, Antioxidants and Stress Tolerance, Tr. Plant Sci., 2002, vol. 7, no. 9, pp. 405-410.
3. Chaitanya K.V., Sundar D., Masilamani S. and Ramachandra R.A., Variation in Heat Stress-Induced Antioxidant Enzyme Activities among Three Mulberry Cultivars, Plant Growth Reg., 2002, vol. 36, no. 2, pp. 175-180.
4. Hernandez J.A., Jimenez A., Mulleniaux P.M. and Sevilla F., Tolerance of Pea (Pisum sativum L.) to Long-Term Salt Stress is Associated with Induction of Antioxidant Defenses, Plant Cell Environ., 2000, vol. 23, pp. 853-862.
5. Fu J. and Huang B., Involvement of Antioxidants and Lipid Peroxidation in the Adaptation of Two Cool-Season Grasses to Localized Drought Stress, Environ. Exp. Bot., 2001, vol. 45, no. 2, pp. 105-114.
6. Neill S.J., Desikan R., Clarke A., Hurst R.D. and Hancock J.T., Hydrogen Peroxide and Nitric Oxide as Signaling Molecules in Plants, J. Exp. Bot., 2002, vol. 53, no. 372, pp. 1237-1247.
7. Bowler C. and Fluhr R., The Role of Calcium and Activated Oxygens as Signals for Controlling Cross-Tolerance, Tr. Plant Sci., 2000, vol. 3, no. 6, pp. 241-244.
8. Chevari S., Chaba I. and Sekey I., Role of Superoxide Dismutase in Cellular Oxidative Processes and Method of Its Determination in Biological Material, Laboratornoe delo, 1985, vol. 11, pp. 578-681.
9. Bilang J. and Sturm A., Cloning and Characterization of Glutathione S-Transferase That Can Be Photolabeled with 5-Azido-Indole-3-Acetic Acid, Plant Physiol., 1995, vol. 109, pp. 253-260.
10. Arnon D.L., Allen M.B. and Whatley L.B., Photosynthesis by Isolated Chloroplasts. Genetic Concept and Comparison of Free Photochemical Reactions, Biochim. Biophys. Acta, 1956, vol. 20, no. 2, pp. 449.
11. Lopez-Delgado H., Dat J.F., Foyer C.H. and Scott I.M., Induction of Thermotolerance in Potato Microplants by Acetylsalicylic Acid and Н2О2, J. Exp. Bot., 1998, vol. 49, no. 321, pp. 713-720.
12. Iavata J. and Tanaka U., Glutathione Reductases “Positive” Spectro-Photometre Assays, Colled. Cresh. Chem. Commun, 1977, vol. 42, no. 3, pp. 1086-1089.
13. Habig W.H., Pabst M.V. and Jacobi W.B., Glutathione S-Transferases, J. Biol. Chem., 1974, vol. 249, pp. 7130-7135.
14. Lowry O.N., Rosenbrough N.J., Tarr A.L. and Randall R.J., Protein Measurement with the Folin Phenol Reagent, J. Biol. Chem., 1951, vol. 193, no. 1, pp. 265-275.
15. Shalygo N.V., Scherbakova R.A., Domanskaya I.N. and Raduyk M.S., Spectrofluorimetric Method of Determination of Oxidized and Reduced Glutathione in Plants, Fiziol. i. biokhim. kul’turnykhrastenii, 2007, vol. 39, no. 3, pp. 1-7.
16. Dospekhov B.A., Metodika polevogo opyta (Method of Field Experimentation), Moscow, 1985.
17. Kosenko E.A., Kaminsky Y.G., Stavrovskaya I.G., Sirota T.V. and Kondrashova M.N., The Stimulatory Effect of Negative Air Ions and Hydrogen Peroxide on the Activity of Superoxide Dismutase, FEBS Letters, 1997, vol. 410, pp. 309-312.
18. Pastori G., Mullineaux P. and Foyer С.. Post-Transcriptional Regulation Prevents Accumulation of Glutathione Reductase Protein and Activity in the Bundle Sheath Cells of Maze, Plant Physiol., 2000, vol. 122, pp. 667-675.
19. Gamparova N.G., Comparative Study of Antioxidant Status of Different Wheat Varieties under the Action of High Temperature, Izv. AN Azerbaijanskoi Respubliki, 2009, no. 1-2, pp. 136-140.
1Baku State University, Baku AZ-1073/1,1148 Azerbaijan, |
Received July 13, 2010
|