doi: 10.15389/agrobiology.2012.3.90eng
УДК 631.46:579.64:581.138.1:581.133:57.044
REACTION OF PEA PLANTS ON INOCULATION BY RHIZOSPHERE 1-AMINOCYCLOPROPANE-1-CARBOXYLATE (ACC) UTILIZING BACTERIA IN THE PRESENCE OF ENDOMYCORRHIZAL FUNGUS Glomus intraradices
A.A. Belimov, S.V. Demchinskaya, V.I. Safronova
In pot experiment with pea genotypes contrasting for efficiency of endomycorrhizal symbiosis (high-efficient genotype 8599 and low-efficient genotype 1025), the plants were grown in the presence or in the absence of endomycorrhizal fungus Glomus intraradices CIAM8 and inoculated with associative bacteria Pseudomonas brassicacearum Am3 or Pseudomonas putida Bm3 containing ACC deaminase. The inoculation of 8599 genotype with Am3 strain increases the biomass of shoots and roots by 60 % for plants without mycorrhiza. In plants with mycorrhiza both strains determined the increased shoot biomass by 40 %, and also root biomass by 40 % (Am3 strain) and by 70 % (Bm3 strain). The influence of mycorrhiza and bacteria on the growth of genotype 1025 is insignificant. After mycorrhization of genotype 8599 the associative bacteria reduced the nitrogen content in shoots by 20 %, but in the absence of mycorrhiza they raised the phosphorus content in genotype 1025 by 25 % (Am3 strain) and by 50 % (Bm3 strain). At a high and similar degree of mycorrhiza development in both pea genotypes the Bm3 strain decreased the number of arbuscles and vesicles in roots of genotype 8599. These results are of interest for more effective application of biopreparations and breeding of the varieties with high symbiotrophity.
Keywords: arbuscular mycorrhiza, ACC deaminase, inoculation, plant-microbe interactions, rhizosphere, symbiosis, Glomus, Pisum sativum, Pseudomonas.
A symbiosis with mycorrhizal fungi the order Glomales (arbuscular mycorrhiza) is an important feature that improves mineral nutrition of many agricultural crops (especially in respect to phosphorus) and increases their productivity. However, artificial selection against high doses of fertilizers weakens symbiotrophic properties of plants (1). This prevents the development of resource-saving and environmental friendly plant growing techniques based on biological principles and the use of plant-microbial symbioses. Such approach was successfully implemented in a new pea variety Triumph developed through a cross of cv Classic with high-symbiotrophic genotype K-8274 (2), though identification and selection of high-symbiotrophic plant genotypes is still out of a proper attention.
Another way to improve endomycorrhizal symbiosis is enhancing positive interactions of symbionts with associative growth-promoting bacteria. It is known that mycorrhizal fungi are actively interact with a wide range of bacteria within a single system of mycosphere (3). In turn, soil bacteria provide beneficial effect on mycorrhizal fungi and symbiotic host-plants by production of bioactive substances, mobilization of soil nutrients, fixation of atmospheric nitrogen and biological control of pathogenic fungi (2, 4). This is used in a promising technique of co-inoculating plants with endomycorrhizal fungi and growth-promoting bacteria. In wheat, an outstanding plant growth was obtained after inoculation of plants with a mixture of bacteria Pseudomonas fluorescens and fungi Glomus mossae (5) or G. intraradices (6). Enterobacter sp. was found to affect growth of alfalfa, contents of nitrogen and phosphorus in plants inoculated with the fungus G. mossae (7). Synergic effect on root growth was observed in clover at dual inoculation with bacteria Brevibacillus brevis and G. mossae (8). However, there were also unsuccessful attempts to improve the efficiency of endomycorrhizal symbiosis in maize (9), barley (10), potatoes (9) and pea (11). This indicates the need in a better study of symbiotrophic interactions and factors determining plant responses to inoculation.
Many associative bacteria contain the enzyme 1-aminocyclo-propane-1-carboxylate (ACC) deaminase, which allows them to reduce the production of ethylene from ACC and promote plant growth. Ethylene is a phytohormone involved in many processes of plant growth and development, and it also regulates the development of symbiotic structures – nodules and endomycorrhiza (12). The positive effect of ACC-utilizing bacteria on plant growth, nutrition, and resistance to abiotic stresses was confirmed by many studies (13, 14). At the same time, their role in plant symbiosis with mycorrhizal fungi is an insufficiently studied issue. It was shown that Ps. putida strain UW4 beneficially affects growth of roots and shoots, leaf area and photosynthetic activity, as well as the frequency of mycorrhizal inclusions in roots of cucumber inoculated with a fungus Gigaspora rosea (15). In these experiments, a mutant strain UW4 not showing ACC-deaminase activity didn’t provide any positive effect on cucumber plants, which indicates the importance of this enzyme for efficient endomycorrhizal symbiosis. At the same time, in pea there were not recorded any additive effects of dual inoculation with ACC-utilizing strain Ps. brassicacearum Am3 and G. intraradices BEG141 (11). Probably, high variation in the performance of symbiosis is the result of complex interactions between micro- and macrosymbionts whose individual sets of properties are involved in formation and functioning of plant-microbial symbiosis.
The purpose of this work was studying the effect of associative bacteria containing ACC deaminase on interactions between host plants and mycorrhizal fungi.
Technique. The experiment was performed using two strains of associative bacteria Ps. brassicacearum Am3 and Ps. putida Bm3, having, respectively, relatively high and low activity of ACC deaminase in vitro (16), and the strain of mycorrhizal fungi G. intraradices CIAM8 forming an effective symbiosis with peas (17). The inoculum of G. intraradices CIAM8 was obtained by growing Sorghum sudanense – source of mycorrhiza – in sterile soil, used then to prepare a soil-root mixture with up to 80% total intensity of mycorrhizal infection. Control variants were inoculated with soil-root mixture not containing mycorrhizal fungi. Experimental plants of pea (Pisum sativum L.) were represented by two genotypes contrasting in growth response to endomycorrhiza: 8599 (highly efficient) and 1025 (low efficient) obtained from the collection of the All-Russia Research and Development Institute of Plant Industry (VIR, St. Petersburg) (17).
Pot experiment was conducted in the summer (June-August, St. Petersburg) in a greenhouse with natural lightning and temperature conditions. The plants were grown in vessels containing 2,5 kg sterilized sod-podzolic soil. Characteristics of the soil: Ctotal – 2,5%; Ntotal - 0.2%, Pmobile - 6 mg P2O5/100 g; Kmobile - 7 mg K2O/100 g; pH of soil extract – 6,0. Fertilizers were used in the following amounts (mg/kg soil): NH4NO3 - 30, KCl - 200, MgSO44 - 60, H3BO3 - 3, MnSO4 - 3, ZnSO4 - 3, Na2MoO4 - 1,5. To provide natural background for plant growth there were introduced nodule bacteria Rhizobium leguminosarum bv. viciae CIAM1026 (the collection of All-Russia Research and Development Institute of Agricultural Microbiology) – a participant of nitrogen-fixing symbiosis with pea (106 cells/g soil).
Pea seeds were sterilized, scarified with concentrated H22SO4 for 30 min and germinated for 3 days. Four seedlings were planted in each vessel; the plants were inoculated with suspensions of associative bacteria (108 cells per seedling). Before that, 25 g soil-root mixture containing G. intraradices CIAM8 (test) or without it (control) was put into each vessel as a layer underlying the seedlings /pot. Soil moisture was maintained at 60-70% of full moisture capacity. The plants were grown 45 days until the beginning of bean formation.
At the end of the experiment the roots were washed in water and stained with aniline blue in lactic acid after discoloration in 15% KOH solution (18). Root samples were examined with a light microscopy to determine the frequency of mycorrhizal colonization (F), abundance of arbuscules in a sample (M), abundance of arbuscules in mycorrhiza-carrying fragments (m), abundance of vesicles in a sample (V) and abundance of vesicles in mycorrhiza-carrying fragments (v) according to Travlo (18). The plants were dried and weighed; total nitrogen content was measured by Kjeldahl method on the automatic analyzer Kjelteck-AUTO (“Tecator”, Sweden), total phosphorus – calorimetrically by color intensity of reduced phospho-molybdenum complex (19).
The experimental data were processed by standard methods with calculation of confidence intervals and Student t-test, as well as by two-factor analysis of variance (Fisher’s F-test) (20).
Results. Inoculation with Ps. brassicacearum strain Am3 provided 60% growth in shoot and root biomass of non-mycorrhizal pea genotype 8599 (Figure).
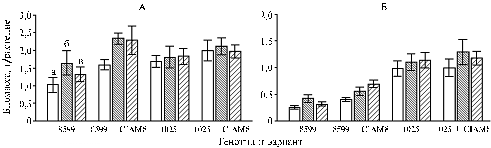 |
Dry biomass of shoots (A) and roots (B) in pea Pisum sativum L. genotypes 8599 and 1025 contrasting in the efficiency of growth response to endomycorrhiza after inoculation with endomycorrhizal fungus Glomus intraradices CIAM8 and associative bacteria: a – control (non-inoculated), b – inoculated with Pseudomonas brassicacearum strain Am3, c – inoculated with Ps. putida strain Bm3. Vertical bars show confidence intervals at Р = 0,05 (pot test, St. Petersburg – Pushkin). |
1. Characteristics of pea Pisum sativum L. genotypes 8599 and 1025 contrasting in the efficiency of growth response to endomycorrhiza (means over all variants with inoculation; pot test, St. Petersburg – Pushkin) |
||
Characteristic |
Genotype 8599 |
Genotype 1025 |
Weight of shoots, g/plant |
1,7 |
1,9* |
Weight of roots, g/plant |
0,45 |
1,14*** |
N content, mg/g biomass |
26,8 |
27,1 |
N accumulation, mg/plant |
45 |
51** |
P content, mg/g biomass |
11,8 |
16,2*** |
P accumulation, mg/plant |
20 |
31*** |
Frequency of mycorrhizal colonization, (F), % |
63 |
60 |
Abundance of arbuscules, %: |
|
|
M |
42 |
44 |
m |
64 |
70 |
Abundance of vesicles, %: |
|
|
V |
28 |
32 |
v |
63 |
69 |
Note. Description of genotypes – see Technique. M and V – resp., abundance of arbuscules and vesicles in a sample, m and v – resp., abundance of arbuscules and vesicles in mycorrhiza-bearing fragment. |
||
In mycorrhiza-colonized plants of this genotype both strains of associative bacteria stimulated the increase in biomass of shoots by 40%, root biomass - by 40% (strain Am3) and 70% (strain Bm3). The influence of bacteria on growth of plants the genotype 1025 wasn’t statistically reliable (Fig.). Mycorrhization of roots by the fungus G. intraradices CIAM8 improved growth of shoots and roots only in the genotype 8599, which mean values were notably different in growth response to mycorrhization compared with the genotype 1025 (Table 1). These results largely coincide with the literature data about pea genotype 8599 as more capable for effective symbiosis with mycorrhizal fungi (17).
| 2. The content (mg/g biomass) and accumulation (mg/ plant) of nitrogen and phosphorus in shoots of pea Pisum sativum L. genotypes 8599 and 1025 contrasting in the efficiency of growth response to endomycorrhiza after inoculation with associative bacteria and endomycorrhizal fungus Glomus intraradices CIAM8 (pot test, St. Petersburg – Pushkin) | ||||
Variant of experiment |
N |
P |
||
content |
accumulation |
content |
accumulation |
|
Genotype 8599 |
||||
Control (non-inoculated) |
26ab |
27a |
14a |
15a |
Pseudomonas brassicacearum Am3 |
24a |
39b |
11a |
18a |
Ps. putida Bm3 |
31b |
41bc |
12a |
16a |
G. intraradicesCIAM8 |
31b |
49cd |
12a |
18a |
G. intraradices CIAM8 + Ps. brassicacearum Am3 |
25a |
57d |
11a |
27b |
G. intraradices CIAM8 + Ps. putida Bm3 |
24a |
56d |
12a |
27b |
Genotype 1025 |
||||
Control (non-inoculated) |
26a |
43a |
12a |
21a |
Ps. brassicacearum Am3 |
25a |
46a |
15ab |
28b |
Ps. putida Bm3 |
26a |
48ab |
18b |
34c |
G. intraradices CIAM8 |
30a |
60c |
19b |
37c |
G. intraradices CIAM8 + Ps. brassicacearum Am3 |
27a |
58c |
18b |
37c |
G. intraradices CIAM8 + Ps. putida Bm3 |
28a |
55cb |
15ab |
29b |
Note. Description of genotypes – see Technique. Superscripts of different Latin letters indicate that inoculated variants of a particular genotype show significant differences for F-test (Р < 0,05). |
||||
Mycorrhization of the genotype 8599 with associative bacteria reduced nitrogen content in shoots by approximately 20%. Probably, this occurred owing to “dilution” with biomass, because nitrogen accumulation in plants inoculated with bacteria wasn’t distinct from that in control variants (Table 2). In plants without a mycorrhiza, this fact can be explained by growth in biomass of shoots promoted by action of bacteria. Elevated levels of phosphorus in plants were reliable only in variants of the genotype 1025 inoculated with monocultures Ps. putida Bm3 and G. intraradices CIAM8 (Table 2), which manifested highest levels of phosphorus accumulation in shoots. Improved phosphorus supply could result from root growth promoted by bacteria in mycorrhiza-carrier genotype 8599, as well as bacteria’s ability to dissolve phosphates not available for plants (16). At the same time, elevated levels of phosphorus in the mycorrhiza-colonized genotype 1025 didn’t contribute to increased plant growth. It is known that phosphorus supply is just one of many positive effects of mycorrhiza on plants necessary for effective integration of symbiotic partners, such as assimilation of other nutrients, exchange of bioactive substances, optimization of water supply, protection from adverse environmental factors, etc. (2). The notable authors’ finding is the potential of ACC-utilizing bacteria to increase phosphorus content in pea; in their earlier studies, these bacteria didn’t’ affect or reduced phosphorus content in different genotypes of pea (15, 21) and rapeseed (22).
3. Characteristics of mycorrhiza in peas Pisum sativum L. genotypes 8599 and 1025 contrasting by intensity of growth response to endomycorrhiza after inoculation with associative bacteria and endomycorrhizal fungus Glomus intraradices CIAM8 (pot test, St. Petersburg – Pushkin)
|
|||||||||
Variant of experiment |
Frequency of mycorrhizal colonization (F), % |
Abundance of arbuscules, % |
Abundance of vesicles, % |
||||||
M |
m |
V |
v |
||||||
Genotype 8599 |
|||||||||
G. intraradicesCIAM8 |
65a |
50a |
76a |
37a |
74a |
||||
G. intraradices CIAM8 + Pseudomonas brassicacearum Am3 |
66a |
42a |
64ab |
26ab |
60ab |
||||
G. intraradices CIAM8 + Ps. putida Bm3 |
58a |
33b |
53b |
20b |
75b |
||||
Genotype 1025 |
|||||||||
G. intraradicesCIAM8 |
66a |
49a |
72a |
33a |
67a |
||||
G. intraradices CIAM8 + Ps. brassicacearum Am3 |
64a |
46a |
70a |
34a |
74a |
||||
G. intraradices CIAM8 + Ps. putida Bm3 |
50a |
38a |
68a |
30a |
68a |
||||
Note. Description of genotypes – see Technique. M and V – resp., abundance of arbuscules and vesicles in a sample, m and v – resp., abundance of arbuscules and vesicles in mycorrhiza-bearing fragment. Superscripts of different Latin letters indicate that inoculated variants of a particular genotype show significant differences for F-test (Р < 0,05). |
|||||||||
A microscopic investigation revealed a high degree of developed mycorrhizal structures in roots of both pea genotypes inoculated with the fungus G. intraradices CIAM8 (Table 3), without any genotypic differences in mycorrhization (Table 1). In variants without introduction into the soil of mycorrhizal fungi there wasn’t observed any mycorrhization of roots. Associative bacteria did not affect the parameters of mycorrhiza formation, except the strain Ps. putida Bm3 reducing the abundance of arbuscules and vesicles in roots of the genotype 8599. Despite this, their plant growth was better than in plants inoculated with the endomycorrhizal fungus (Fig.). Previously, a similar pattern of decrease in mycorrhization of roots influenced by bacteria while a simultaneously enhanced plant growth was observed in experiments with wheat, associative bacterium Ps. fluorescens and fungus G. mossae (6). It was also shown that in mycorrhiza-colonized alfalfa plants enhanced growth and increase in nitrogen and phosphorus contents promoted by the strain Enterobacter sp. wasn’t accompanied by increased mycorrhization of roots (7). It should be noted that intensity of mycorrhization wasn’t always correlated with growth responses of plants to endomycorrhizal fungi, in fact, in some cases a fungus can show parasitic properties (9, 23).
Positive effects of the studied bacteria was observed in the genotype 8599 more efficiently interacting with endomycorrhizal fungus. In this case, growth stimulation was more pronounced in mycorrhiza-colonized plants. Earlier, in rapeseed the authors observed termination of growth stimulation by Ps. putida Bm3 under phosphorus deficiency; the latter suppressed ethylene biosynthesis in plants and neglected the effect of bacterial ACC deaminase on this phytohormone and plant growth (22). However, the phosphorus content and the effect of G. intraradices CIAM8 on phosphorus utilization in pea the genotype 8599 responsive to inoculation was lower than those of the genotype 1025 (Table 2) while a similar performance of mycorrhizal structures (Table 1). Therefore genotypic differences in pea response to ACC-recycling bacteria can’t be explained by improved phosphorus nutrition of mycorrhiza-colonized plants. The importance of ACC-deaminase of studied bacterial strains for interaction with plants was shown in the earlier authors’ study of ethylene-sensitive mutant pea E2 (sym5) (16) and mutant Ps. brassicacearum Am3 with defective gene for ACC-deaminase (24). In vitro the strain Ps. brassicacearum Am3 has higher activity of ACC-deaminase (2,5 times) than Ps. putida Bm3 (16). In the authors’ experiments on pea only this strain stimulated growth in plant not having a mycorrhiza, which indirectly shows the involvement of ACC-deaminase in promotion of plant growth. Probably, this mycorrhizal fungus enhanced the intensity of ethylene biosynthesis or plants’ sensitivity to ethylene (11), which positively affected their response to ACC-recycling bacteria. Testing this hypothesis necessitates a comparison of studied genotypes in respect to production and sensitivity to ethylene in plants with mycorrhiza or without it.
Thus, in this study it was shown for the first time the effect of associative ACC-utilizing bacteria on growth and mineral supply of pea depending on endomycorrhiza present or absent in roots. Mycorrhization of plants enhanced growth-promoting action of the bacteria. Manifestation of additive effects of ACC-utilizing bacteria and endomycorrhizal fungus on growth and mineral supply of pea is largely determined by its genotype. Responsiveness of plants to inoculation with ACC-utilizing bacteria and endomycorrhizal fungi can be assumed as interrelated features. Discovering such relationship could be useful for selection of varieties with a high symbiotic potential to both growth-stimulating bacteria and mycorrhizal fungi.
REFERENCES
1. Tikhonovich I.A. and Provorov N.A., Simbiozy rastenii i mikroorganizmov: molekulyarnaya genetika agrosistem buduschego (Symbioses of Plants and Microorganisms: Molecular Genetics of Future Agroecosystems), St. Petersburg, 2009.
2. Shtark O.Y., Borisov A.Y., Zhukov V.A., Provorov N.A. and Tikhonovich I.A., Intimate Associations of Beneficial Soil Microbes with the Host Plants, in Soil Microbiology and Sustainable Crop Production, The Netherlands, Dordrecht: Springer Science+Business Media, B.V., 2010, pp. 119-196.
3. De Boer W., Folman L.V., Summerbell R.C. and Boddy L., Living in a Fungal World: Impact of Fungi on Soil Bacterial Niche Development, FEMS Microbiol. Rev., 2005, vol. 29, pp. 795-811.
4. Frey-Klett P., Garbaye J. and Tarkka M., The Mycorrhiza Helper Bacteria Revisited, New Phytol., 2007, vol. 176, pp. 22-36.
5. Behn O., Influence of Pseudomonas fluorescens and Arbuscular Mycorrhiza on the Growth, Yield, Quality and Resistance of Wheat Infected with Gaeumannomyces graminis, J. Plant Dis. Protect., 2008, vol. 115, pp. 4-8.
6. Jaderlund L., Arthurson V., Granhall U. and Jansson J.K., Specific Interactions between Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Bacteria: as Revealed by Different Combinations, FEMS Microbiol. Lett., 2008, vol. 287, pp. 174-180.
7. Toro M., Azcon R. and Barea J.M., The Use of Isotopic Dilution Techniques to Evaluate the Interactive Effects of Rhizobium Genotype, Mycorrhizal Fungi, Phosphatesolubilizing Rhizobacteria and Rock Phosphate on Nitrogen and Phosphorus Acquisition by Medicago sativa, New Phytol., 1998, vol. 138, pp. 265-273.
8. Vivas A., Barea J.M. and Azcon R., Interactive Effect of Brevibacillus brevis and Glomus mosseae, Both Isolated from Cd Contaminated Soil, on Plant Growth, Physiological Mycorrhizal Fungal Characteristics and Soil Enzymatic Activities in Cd Polluted Soil, Environ. Pollut., 2005, vol. 134, pp. 257-266.
9. Vosatka M. and Gryndler M., Treatment with Culture Fractions from Pseudomonas putida Modifies the Development of Glomus fistulosum Mycorrhiza and the Response of Potato and Maize Plants to Inoculation, Appl. Soil Ecol., 1999, vol. 11, pp. 245-251.
10. Belimov A.A., Serebrennikova N.V. and Stepanok V.V., Interaction of Associative Bacteria and an Endomycorrhizal Fungus with Barley upon Dual Inoculation, Mikrobiologiya, 1999, vol. 68, no. 1, pp. 122-126.
11. Engqvist L.G., Mårtensson A., Orlowska E., Turnau K., Belimov A.A., Borisov A.Y. and Gianinazzi-Pearson V., For a Successful Pea Production on Polluted Soils, Inoculation with Beneficial Microbes Requires Active Interaction between the Microbial Components and the Plant, Acta Agric. Scand., B, 2006, vol. 56, pp. 9-16.
12. Guinel F.C. and Geil R.D., A Model for the Development of the Rhizobial and Arbuscular Mycorrhizal Symbioses in Legumes and Its Use to Understand the Roles of Ethylene in the Establishment of These Two Symbioses, Can. J. Bot., 2002, vol. 80, pp. 695-720.
13. Belimov A.A. and Safronova V.I., ACC Deaminase and Plant – Bacteria Interactions (Review), S.-kh. biol., 2011, vol. 3. pp. 23-28.
14. Glick B.R., Cheng Z., Czarny J. and Duan J., Promotion of Plant Growth by ACC Deaminase-Producing Soil Bacteria, Eur. J. Plant Pathol., 2007, vol. 119, pp. 329-339.
15. Gamalero E., Berta G., Massa N., Glick B.R. and Lingua G., Interactions between Pseudomonas putida UW4 and Gigaspora rosea BEG9 and Their Consequences for the Growth of Cucumber Under Salt-Stress Conditions, J. Appl. Microbiol., 2010, vol. 108, pp. 236-245.
16. Belimov A.A., Safronova V.I., Sergeyeva T.A., Egorova T.N., Matveyeva V.A., Tsyganov V.E., Borisov A.Y., Tikhonovich I.A., Kluge C., Preisfeld A., Dietz K.-J. and Stepanok V.V., Characterization of Plant Growth-Promoting Rhizobacteria Isolated from Polluted Soils and Containing 1-Aminocyclopropane-1-Carboxylate Deaminase, Can. J. Microbiol., 2001, vol. 47, pp. 642-652.
17. Jakobi L.M., Kukalev A.S., Ushakov K.V., Tsyganov V.E., Naumkina T.S., Provorov N.A., Borisov A.Yu. and Tikhonovich I.A., Polymorphism of Garden Pea for the Efficiency of Symbiosis with Endomycorrhizal Fungus Glomus sp. under the Conditions of Rhizobia Inoculation, S.-kh. biol., 2000, vol. 3, pp. 94-102.
18. Zol’nikova N.V. and Vorob’yev N.I., Metody issledovaniya gribov, obrazuyuschikh s rasteniyami mikorizu arbuskulyarno-vezikulyarnogo tipa (Methods for Studying Fungi that Form Arbuscular-Vesicular Mycorrhiza with Plants), St. Petersburg, 1992.
19. Voskresenskaya O.L., Alyabysheva E.A. and Polovnikova M.G., Bol’shoy praktikum po bioekologii. Ch. 1, (Practical Guidelines on Bioecology. Part 1), Yoshkar-Ola, 2006.
20. Lakin G.F., Biometriya (Biometrics), Moscow, 1990.
21. Safronova V.I., Stepanok V.V., Engqvist G.L., Alekseyev Y.V. and Belimov A.A., Root-Associated Bacteria Containing 1-Aminocyclopropane-1-Carboxylate Deaminase Improve Growth and Nutrient Uptake by Pea Genotypes Cultivated in Cadmium Supplemented Soil, Biol. Fertil. Soils, 2006, vol. 42, pp. 267-272.
22. Belimov A.A., Safronova V.I. and Mimura T., Response of Spring Rape to Inoculation with Plant Growth-Promoting Rhizobacteria Containing 1-Aminocyclopropane-1-Carboxylate Deaminase Depends on Nutrient Status of the Plant, Can. J. Microbiol., 2002, vol. 48, pp. 189-199.
23. Huiying L., Smith F.A., Dickson S., Holloway R.E. and Smith S.E., Plant Growth Depressions in Arbuscular Mycorrhizal Symbioses: Not Just Caused by Carbon Drain?, New Phytol., 2008, vol. 178, pp. 852-862.
24. Belimov A.A., Dodd I.C., Safronova V.I., Hontzeas N. and Davies W.J., Strain Am3 Containing 1-Aminocyclopropane-1-Carboxylate Deaminase Can Show Both Pathogenic and Growth-Promoting Properties in Its Interaction with Tomato, J. Exp. Bot., 2007, vol. 58, pp. 1485-1495.
All-Russia Research and Development Institute of Agricultural Microbiology, RAAS, St. Petersburg – Pushkin 196608, Russia, |
Received February 7, 2012
|













