ÓÄÊ 633.111.1:581.132:631.811.91:58.02
FUNCTIONAL CHANGES OF PHOTOSYNTHETIC APPARATUS IN WHEAT PLANTS AT WATER STRESS AGAINST THE BACKGROUND OF NaCl
A.A. Ivanov
O2-gas exchange, the pigments contents and water status in leaves of 2-week wheat seed-lings (Triticum aestivum L.) during development of water stress at various concentrations of NaCl in soil were investigated. The decrease in rate of photosynthetic oxygen evolution, the relative water content (RWC) and chlorophyll content in leaves under drought conditions was shown only after achievement of the water contents in soil below the certain critical level. NaCl negatively affected photosynthetic and physiological parameters at sufficient water contents in soil àt strong water stress.
Key words: wheat leaf rust, brown rust of wheat, pathogenesis, Thatcher isogenic lines, Lr-genes.
defined as a short-term stressor. When moisture content declines in soil, its water potential slightly changes to reach a certain critical level, after which it sharply increases. Such a transition usually indicates the beginning of water stress, when the amount of plant-available water significantly decreases.
Under these conditions, the protective mechanisms and effectiveness of recovery system after normalization of water regime become of the highest importance (1). One way to combat stress is accumulation in vacuoles of osmotically active substances (2) allowing to level the ratio of water vapor pressure in a system “soil - plant”. The osmotical function can be performed by soil mineral salts, including NaCl.
The increased content of a soil NaCl, which is not involved in plant metabolism, leads to inhibition of almost all vital functions and is considered as a negative factor. Each type of soil contains a certain content of sodium ions, which allows to estimate the effect of salt factor as a long-term impact leading to a global reorganization of plant metabolism and structure - functional systems. Plant species differ in their sensitivity to salt stress, but the common response to saline conditions is a strong growth retardation (3). The observed suppression of growth is often associated with a decrease in the rate of photosynthesis, primarily as a result of changes in activity of photosystem II (4). However, the literature data about salinity effects on photosynthesis are ambiguous. It has been reported that salt stress suppresses photosynthetic activity (5-7), while other data describe no significant effect of NaCl on the activity of photosynthetic apparatus (8-11).
Under natural conditions, plants are often subjected to simultaneous influence of several stressors, and the result of their interaction is unpredictable - from mutual reinforcement to antagonistic effect (12). It is known that small impact of one stressor contributes to better adaptation of plants to other adverse environmental factors (13, 14). It is possible, that small concentrations of NaCl cause a positive effect on wheat plants exposed to the action of other stressors. It was shown, that activity of photosynthetic apparatus in plants is one of the most stressor-sensitive parameters (15, 16). Though, the combined effect of salt and water stress had a little attention of researchers, while the importance of such studies is obvious owing to the expanding salinization of agricultural lands, much of which is located in areas with low water availability (17).
The purpose of our work was identification of functional changes in photosynthetic apparatus of wheat seedlings under conditions of gradual dehydration of soil at different contents of NaCl.
Methods. Wheat (Triticum aestivum L.) plants were grown in vessels (volume 0,5 l) on a substrate - the river sand (well-washed free of any soluble compounds) in the climatic chamber ShKV-1 (Russia). Illumination at the level of the upper leaves - 450 mkmolLm-2Ls-1 (measured with a LI-250 QSX-01 quantum meter (“Li-Cor”, USA). Cultivation temperature - 20/17 °C (day / night) with 12 - hour photoperiod.
The experiment was performed upon the 10-day-old wheat seedlings at a phase of 2 “true” leaves, grown in salt-free conditions with a maximum moistening of substrate; total moisture capacity (TM) of the substrate - 155 ml water per1 kg of sand. Then, different quantities of 2.5 M NaCl solution and distilled water (up to a final concentration of NaCl 0,05; 0,1; 0,2 and 0,3 M) were added into each vessel; doing so, vessels weight was leveled to an equal value considering substrate TC. After that, watering in all variants was stopped. A control – variant without adding NaCl. Experiments were carried out over several days with a gradual decrease of water content in soil.
Photosynthetic O2 release in samples cut from wheat leaves (18) was measured in 5-milliliter thermostatted cell with a standard platinum Clarke electrode, at light intensity 1200 umol fotonsLm-2Ls-1 and temperature 28 °C. 50 mM K-phosphate buffer (pH 7.5) was used. Before the measurements, the excessive amount of sodium bicarbonate (0,1 ml 0,5 M NaHCO3) was added to the solution as a carbon source supply for photosynthetic reactions. The rate of photosynthesis was measured on the polarograph LP-7E (Czech Republic), starting from measurements of light-independent respiration after 15 min of incubation in the dark.
Relative water content (RWC) was determined in segments of leaves (20 mm) as the ratio of full crude (FW) and dry (DW) biomass according to the equation:
RWC = 100 ‡ (FW - DW) / (TW - DW).
The value of TW (total biomass of leaves at full water saturation) was measured after placing the leaves pieces into distilled water at 20 °C and soft lightening for 24 h, DW - after drying the leaves in thermostat at 75 oC for 48 h (19).
To determine the content of pigments, the leaves were homogenized in a porcelain mortar with addition of CaCO3, and the pigments were extracted in 80% acetone; the measurements were performed on a single-beam spectrophotometer GenesysTM 10uv («Thermo Spectronic», Lanham, MD, USA) at wavelengths of 470, 646 and 663 nm, applying an extinction ratio of 80% acetone (20).
Experiments were performed in 3-5 biological replications. Processing of data was performed using the statistical program. The table shows average values and standard deviations for 3-5 independent measurements. In all the experiments, measurements were made in 3 analytical replicates.
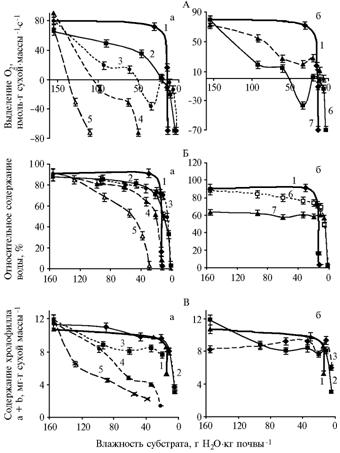
Results. Dynamics of change in substrate relative humidity is presented in the table. In the control (without NaCl), the rate of photosynthetic O2 release by wheat leaves remained unchanged up to a certain critical level of substrate moistening (Fig. A, a). A further decrease of water content in substrate led to a sharp decrease in photosynthetic activity and plant death. It is possible, that low moisture content in soil sharply increases water potential of substrate, which results in reduction of amount of plant-available water. The addition of 0,05 M NaCl (initial concentration) led to the decrease in O2 release by leaves at normal substrate moistening, but allowed to maintain photosynthesis in plants in the conditions of water content in substrate below a critical level for the control variant (about 10% TM). The positive effect of NaCl started to be manifested particularly at very small water content in substrate. The maximum positive effect at low content of water was observed with increasing the concentration of NaCl to 0.1 M.
The presence of salt increases water-holding capacity of a substrate, though it also raises its osmotic potential, which elevates water deficit during a drought. To some extent, plants are able to compensate the increasing difference between water potentials in a system substrate / plant by the transport of sodium ions into cell vacuoles. Too high content of NaCl in substrate (0,3-0,4 M) leads to the beginning of salt stress, resulting in clearly marked reduction in the rate of photosynthesis even under normal moisture content in substrate.
Dynamics of change in relative water content in substrate (% of TM) in variants of the experiment, depending on concentration of NaCl in substrate during the 216-hour draught |
|||||
Time, h |
Í20 (control) |
0,05 Ì |
0,1 Ì |
0,2 Ì |
0,3 Ì |
0 |
100 |
100 |
100 |
100 |
100 |
24 |
42,6 |
69,2 |
73,8 |
77,5 |
86,6 |
48 |
21,4 |
49,7 |
58,3 |
62,1 |
74,4 |
72 |
9,2 |
29,9 |
38,9 |
41,5 |
56,6 |
96 |
3,5 |
13,9 |
21,8 |
25,2 |
35,5 |
120 |
0,4 |
7,1 |
13,0 |
15,1 |
24,6 |
144 |
0,1 |
3,0 |
7,3 |
8,8 |
15,1 |
168 |
0 |
0,8 |
4,2 |
5,8 |
10,2 |
192 |
0 |
0,1 |
1,8 |
3,3 |
6,3 |
216 |
0 |
0 |
0,5 |
1,5 |
3,6 |
|
Photosynthetic O2 release (A A), relative water content in tissues (Á B) and the content of chlorophyll a + b (Â C) depending on water content in substrate during the development of water stress in the 1st leaf of wheat seedlings against a background of various concentrations of NaCl (à a) and in the 1st and 2nd leaves at presence of 0.1 M NaCl in substrate (á b): Note: |
The similar effect was observed when determining water content in leaves at different moisture and salt contents in substrate (see Fig. B, a). The presence of NaCl at the initial concentration of 0,05-0,1 M caused a somewhat reduction of water content in plants under normal conditions (most likely as a result of osmotic effects of salt), but also contributed to maintaining their water balance even at low water content in substrate. Further increase in substrate salinity (up to 0,3-0,4 M) initiated salt stress, which elevated drought effects.
The content of chlorophyll a + b and RWC in leaves was decreasing synchronously and insignificantly changed at low content of salt (see Fig. B, a). High (0,3-0,4 M) concentrations of NaCl promoted rapid destruction of the pigment, even in conditions of normal substrate moistening. The ratio of chlorophyll a / b contents remained almost unchanged throughout the experiment in all the variants, even in early period of sharp decrease in a content of pigments at low water content. This fact can be explained by relatively rapid dehydration of plants, leading to chloroplasts inactivation.
As it has been reported by some authors, a high substrate salinity damages plants photosynthetic apparatus (6, 7), whereas the introduction of small amounts of NaCl increases its thermal stability (15, 16). A similar influence has been shown for water stress, whose mechanism is largely similar to that of salinity (21). It is also possible, that the increasing thermal stability of plants subjected to saline pre-processing and water stress is the part of protection mechanism against osmotic stress, or a certain common protective mechanism probably controlled at the genetic level.
The processes underlying the protective effect of NaCl for the photosynthetic apparatus are unclear. Perhaps, NaCl triggers the formation of substances increasing stability of thylakoid membranes. Osmolites (glycine-betaine etc.) are able to accumulation in chloroplasts thus raising the stability of photosynthetic apparatus at temperature stress (22-24). The existence of similar mechanism is possible at water stress too. Indeed, under the salt (25, 26) or water (27) stress, plants accumulate sugars and proteins. Fast adaptation of plants to changing environmental conditions have the great physiological significance as it increases their chances for survival.
The study of photosynthesis in leaves of different ages in one plant is of the particular attention. In adverse conditions, old leaves die first (1st leaves, or the 1st tier from the ground), while the younger leaves retain high activity (2nd leaves, or the 2nd tier from the ground). In our experiments, the development of water stress promoted almost identical changes in photosynthetic O2 release rates in leaves of both age groups. However, at small content of NaCl (0.1 M) in substrate, the younger leaves demonstrated higher photosynthetic activity during all periods of drought (see Fig. A, B). Unlike the older leaves, young leaves contain less chlorophyll (see Fig. In, b) and have less water content in tissues. Therefore, answering the question about origins of high photosynthetic activity in young leaves under salinity conditions, this is probably not the better water saturation of tissues (see Fig. B, B), but the redistribution of NaCl transport within a plant. Sodium ions contribute to the maintenance of water balance in a whole plant under drought conditions, but since NaCl is being transported primarily in older leaves, the younger ones are in better conditions as they are subjected to less inhibitory effect of salt. In a control (no salt in substrate), no difference in photosynthetic activity in leaves of different ages was identified.
Thus, wheat plants under conditions of acute water shortage, have more chances to survive against a background of small (not causing severe salt stress) amounts of NaCl in substrate. The narrow range of soil salinity causing a positive effect in conditions of water deficit has been established - from 0,05 to 0,1 M (initial concentration at the maximum substrate wetting). At presence of NaCl, plants maintain viability despite the sharp increase in water potential of substrate at low moisture content, which has led to plant death in the salt-free version.
1. Y o r d a n o v I., V e l i k o v a V., T s o n e v T. Plant responses to drought, acclimation, and stress tolerance. Photosynthetica, 2000, 38: 171-186.
2. S a c h s M.M., H o T.-H.D. Alteration of gene expression during environmental stress in plants. Ann. Rev. Plant Physiol., 1986, 37: 363-376.
3. M u n n s R., T e r m a a t A. Whole plant responses to salinity. Aust. J. Plant Physiol., 1986, 13: 143-160.
4. B a k e r N.R. Possible role of photosystem II in environmental perturbations of photosynthesis. Physiologia Plantarum, 1991, 81: 563-570.
5. B o n g i G., L o r e t o F. Gas-exchange properties of salt-stressed olive (Olea europea L.) leaves. Plant Physiol., 1989, 90: 1408-1416.
6. M i s h r a S.K., S u b r a h m a n y a m D., S i n g h a l G.S. Interactionship between salt and light stress on the primary process of photosynthesis. J. Plant Physiol., 1991, 138: 92-96.
7. M a s o j i d e k J., H a l l D.O. Salinity and drought stresses are PSII thermostability in salt-adapted plants 859 amplified by high irradiance in sorghum. Photosynthetica, 1992, 27: 159-171.
8. R o b i n s o n S.P., D o w n t o n W.J.S., M i l l h o u s e J. Photosynthesis and ion content of leaves and isolated chloroplasts of salt-stressed spinach. Plant Physiol., 1983, 73: 238-242.
9. B r u g n o l i E., B j o r k m a n O. Growth of cotton under continuous salinity stress: influence on allocation pattern, stomatal and non-stomatal components of photosynthesis and dissipation of excess light energy. Planta, 1992, 187: 335-345.
10. M o r a l e s F., A b a d i a A., G o m e z - A p a r i s J., A b a d i a J. Effects of combined NaCl and CaCl2 salinity on photosynthetic parameters of barley grown in nutrient solution. Physiologia Plantarum, 1992, 86: 419-426.
11. A b a d i a A., B e l k o h o d j a R., M o r a l e s F., A b a d i a J. Effects of salinity on the photosynthetic pigment composition of barley (Hordeum vulgare L.) growth under a triple-line-source sprinkler system in the field. J. Plant Physiol., 1999, 154: 392-400.
12. O s m o n d C.B., A u s t i n M.P., B e r r y J.A., B i l l i n g s W.D., B o y e r J.S., D a-
c e y W.J.H., N o b e l P.S., S m i t h S.D., W i n n e r W.E. Stress physiology and the distribution of plants. BioScience, 1986, 37: 38-48.
13. L u C., Q i u N., W a n g B., Z h a n g J. Salinity treatment shows no effects on photosystem II photochemistry, but increases the resistance of photosystem II to heat stress in halophyte Suaeda salsa. J. Exp. Bot., 2003, 54: 851-860.
14. W e n X., Q i u N., L u Q., L u C. Enhanced thermotolerance of photosystem II in salt-adapted plants of the halophyte Artemisia anethifolia. Planta, 2005, 220: 486-497.
15. B e l k h o d j a R., M o r a l e s F., A b a d i a A., G o m e z - A p a r i s i J., A b a d i a J. Chlorophyll fluorescence as a possible tool for salinity tolerance screening in barley (Hordeum vulgare L.). Plant Physiol., 1994, 104: 667-673.
16. E v e r a r d J.D., G u c c i R., K a n n S.C., F l o r e J.A., L o e s c h e r W.H. Gas exchange and carbon partitioning in the leaves of celery (Apium graveolens L.) at various levels of root zone salinity. Plant Physiol., 1994, 106: 281-292.
17. E p s t e i n E., N o r l y n J.O., R u s h D.W., K i n g s b u r y R.W., K e l l e y D.B., C u n n i n g h a m G.A., W r o n a A.F. Saline culture of crops. Science, 1980, 210: 399-404.
18. B i l’ K.Ya., F o m i n a I.R., T s e n o v a E.N. Effects of nitrogen nutrition on photosynthetic enzyme activities, type of photosynthates and photosystem 2 activity in maize leaves. Photosynthetica, 1985, 19: 216-220.
19. P a r d o s s i A., V e r n i e r i P., T o g n o n i F. Involvement of abscisic acid in regulating water status in Phaseolus vulgaris L. during chilling. Plant Physiol., 1992, 100: 1243-1250.
20. L i c h t e n t h a l e r H.K. Chlorophylls and carotenoids: pigments of photosynthetic biomenbranes. Meth. Enzymol., 1987, 148: 350-382.
21. L u C., Z h a n g J. Effects of water stress on photosystem II photochemistry and its thermostability in wheat plants. J. Exp. Bot., 1999, 50: 1199-1206.
22. M u r a t a N., M o h a n t y P.S., H a y a s h i H., P a p a g e o r g i o u G.C. Glycinebetaine stabilizes the association of extrinsic proteins with the photosynthetic oxygen-evolving complex. FEBS Lett., 1992, 296: 187-189.
23. P a p a g e o r g i o u G.C., M u r a t a N. The unusually strong stabilizing effects of glycinebetaine on the structure and function of the oxygen-evolving photosystem II complex. Photosynt. Res., 1995, 44: 243-252.
24. A l l a k h v e r d i e v S.I., F e y z i e v Y.M., A h m e d A., H a y a s h i H., A l i e v J.A., K l i m o v V.V., M u r a t a N., C a r p e n t i e r R. Stabilization of oxygen evolution and primary electron transport reactions in photosystem II against heat stress with glycinebetaine and sucrose. J. Photochem. Photobiol, B: Biol., 1996, 34: 149-157.
25. G r e e n w a y H., M u n n s R. Mechanisms of salt tolerance in nonhalophytes. Ann. Rev. Plant Physiol., 1980, 31: 149-190.
26. I m a m u l H u q S.M., L a r h e r F. Osmoregulation in higher plants: effect of maintaining a constant Na:Ca ratio on the growth, ion balance and organic solute status of NaCl-stressed cowpea (Vigna sinensis L.). Zeitschrift fur Pflanzenphysiologie, 1984, 113: 163-176.
27. S e e m a n n J.R., D o w n t o n W.J.S., B e r r y J.A. Temperature and leaf osmotic potential as factors in the acclimation of photosynthesis to high temperature in desert plants. Plant Physiol., 1986, 80: 926-930.
Institute of Fundamental Problems of Biology, Russian Academy of Sciences, |
Received March 25, 2009
|