doi: 10.15389/agrobiology.2012.2.87eng
УДК 636.598:619:579.62:579.887.111:579.25
DETECTION AND GENETIC IDENTIFICATION OF Mycoplasma sp. 1220 IN GEESE IN THE RUSSIAN FEDERATION AND UKRAINE
A.V. Sprygin1, D.V. Volokhov2, V.N. Irza1, V.V. Drygin1
The authors investigated samples of pathological material from 47 domestic geese with clinical presentation of inflammation of phallus, cloaca and oviduct obtained from two goose farms in Russia and one farm in Ukraine. It is known that in chickens similar clinical manifestations may be caused by infection with Mycoplasma gallisepticum, but in geese, however, this may be due to infection with opportunistic bacteria from Neisseria and Candida genera, and also with Mycoplasma sp. 1220, a recently described opportunistic mycoplasma in domestic geese. Using PCR, we detected no Mycoplasma gallisepticum in the studied birds. However, at the same time, these tested samples were positive for the rpoB, dnaE, fusA, pyk genes of Mycoplasma sp. 1220. Sequencing and analysis of these amplicons have demonstrated their 98-99 % nucleotide similarity with corresponding sequences from GenBank for Mycoplasma sp. 1220, the Mycoplasma species previously isolated in Hungary from domestic geese with clinical signs of pathological changes in the reproductive system (a species name of the microorganism has not yet assigned). Our study represents the first evidence of detection and genetic identification of Mycoplasma sp. 1220 in domestic geese in the Russian Federation and Ukraine.
Keywords: mycoplasma, inflammation, geese, gene identification.
Mycoplasma is known as a causative agent of acute and chronic infectious diseases of poultry resulting in a decrease of egg production, weight loss, livestock mortality and high economic damage to poultry farms (1-6). The efficiency of combating mycoplasmosis significantly depends on early detection and identification of the agent (5, 7).
First data about mycoplasma in geese were reported in the scientific literature a few decades ago (8-13). Mycoplasmosis is clinically expressed in birds as inflammation of air sacs, peritonitis, salpingitis, excessive embryonic mortality, inflammation of phallus, cloaca, ovary and oviduct (5, 6, 8, 10, 14, 15). Mycoplasma gallisepticum was found to cause inflammation of the oviduct in laying hens (16), and in geese was observed a transovarial infestation (11).
Previously, the reduce in egg production, increased embryonic mortality, inflammation of digestive and reproductive organs in geese were associated with mycotoxins present in feed (17, 18). However (in addition to possible mycotoxicoses), epizootological researches and experiments have revealed the infectious nature of this disease whose most probable agents are the genera Neiseria and/or Candida (19-21). A common set of clinical symptoms was recorded in geese in Israel (22), Hungary (19, 20), and the USSR (21); however, most of the reported cases didn’t include any complex investigations on etiology of the disease.
It ought to be remarked that M. anseris, M. cloacale and Acholeplasma spp. are representatives of normal microflora of geese (12, 23). Pathogenicity of these species for goose embryos, 1-day goslings or she-geese in early reproductive period was observed only in experimental conditions under high doses of infection, but never seen at natural infestation (8, 10, 24-27). In 1983 in Hungary, the new type of bacterial agent denoted as Mycoplasma sp. 1220 was isolated from a he-goose with clinical inflammation of the phallus (14, 26). Biochemical and serological properties of this pathogen were studied, which allowed considering this isolate as a new species (14, 25-27) tentatively named Mycoplasma anserisalpingitis (DV Volokhov and L. Stipkovits, personal report). Since this species’ name hasn’t been officially assigned yet, the denotation Mycoplasma sp. 1220 was used in this study as a species name
Mycoplasma sp. 1220 is a non-moving pleomorphic prokaryotic microorganisms capable to penetrate through filters of 0,22 micron pore size and grow in the temperature range from +30 to +40 °C in both aerobic and anaerobic conditions. It ferments glucose and fructose (fructose is preferable and fermented faster than glucose), not able to utilize arginine, esculin and urea, shows phosphatase activity, for growth in vitro it requires sterols or serum. Morphological and cultural characteristics of Mycoplasma sp. 1220 are almost indistinguishable from M. anatis earlier described in domestic and wild ducks, and rarely recorded in geese (12). Mycoplasma sp. 1220 can be easily isolated from a fresh pathological material obtained from geese (swabs and scrapings from mucous membranes of oviduct, cloaca, lymph from the phallus, etc.) and grown on the media Hayflick, Frey and PPLO (”Becton-Dickinson”, Franklin Lake NJ, USA) supplemented with 0,5-1,0% D-fructose and 15-20% horse serum (D.V. Volokhov, personal report).
Various mycoplasma species can be differentiated mainly by using specific antisera to each species in reactions of cross-inhibition of growth and/or metabolic activity (14, 28) along with genetic identification based on genes for 16S-rRNA and b-subunit of DNA-dependent RNA-polymerase rpoB (29).
Earlier, the authors had established Mycoplasma sp. 1220 isolated in Hungary as a member of so-called phylogenetic cluster M. synoviae (29) quite similar to M. anatis – non-pathogenic and/or conditionally pathogenic species present in domestic and wild ducks (12, 30). Mycoplasma sp. 1220 and M. anatis have a very small difference in species-specific mutations of nucleotide sequences of 16S-rRNA genes, which factor doesn’t allow discriminating these species in isolates obtained from geese, while the presence of significant differences in nucleotide sequences of rpoB gene for b-subunit of DNA-dependent RNA polymerase allows an accurate identification of these species (29).
Transmission of the infection caused by Mycoplasma sp. 1220 haven’t been studied yet. Dr. L. Stipkovits (Hungary), who first described this species, believed that geese are infected by it during coitus and/or transovarially to an offspring, while both males and females can be infected with this agent for a long period without any clinical expression (personal report).
Pathogenic properties of Mycoplasma sp. 1220 were studied and represented in 1980-1990ies by a group of Hungarian veterinary specialists (14, 25-27). However, many issues of Mycoplasma sp. 1220 still remain insufficiently known – its interaction with other microorganisms and their involvement in pathogenic process, molecular basis of pathogenesis, antibiotic resistance and persistence in geese during antibiotic therapy and after it . At the same time, clarifying these facts can help to improve the effectiveness of curing the disease. Today, prevention and medication of mycoplasmosis include mainly antibiotic therapy (tylosin, tiamulin, linco-spectin) (31), improving housing conditions and artificial insemination.
The purpose of this study was isolation and genetic identification of Mycoplasma sp. 1220 in domestic geese with clinical symptoms of inflammation of phallus, cloaca and oviduct.
Technique. Samples for analysis (laryngeal and cloacal swabs, pathological material from compulsory slaughtered geese with clinical symptoms of salpingitis, inflammation of cloaca and phallus) were obtained from poultry farms during 2009-2011. Pathological material included the fragments of internal organs (liver, spleen, intestine, gonads) was used to prepare a total sample from each individual for subsequent DNA extraction. 47 individual samples were obtained from sick geese kept in farms of different regions: 5 – in Ural Federal District and 8 – in Volga Federal District of Russia, and 34 – in Ukraine.
There were also investigated 15 laryngeal swabs from clinically healthy geese from a geese farm of the Central Federal District of Russia. The samples of pathological material were placed in cryovials and stored at -70° C. To exclude the presence of other infections with similar clinical presentation, the samples were analyzed using classical bacteriological culture tests to reveal Pasteurella multocida, Salmonella spp., Neisseria spp. and Escherichia coli (21, 32). The blood serum was tested for the presence of antibodies to avian influenza and Newcastle disease using corresponding serological test kits (”Federal Center for Animal Health”, Russia) according to the manufacturer's instructions (7). The presence of M. gallisepticum and M. synoviae in the samples was determined by PCR (33).
Total DNA was extracted from the samples using DNA-sorb kit (”AmpliSens”, Russia) according to the manufacturer's recommendations.
Genetic detection and identification of Mycoplasma sp. 1220 in samples was performed by a one-round PCR using primer pairs for fragments of genes for b-subunit of DNA-dependent RNA polymerase (rpoB), a-subunit of DNA polymerase III (dnaE), elongation factor (fusA), and pyruvate kinase (pyk). PCR was performed on the device Tertsik (“DNA technology”, Moscow) using reagents and Taq-polymerase produced by ”Promega” Co. (USA). A final volume of reaction mixture was 25 ul, the concentration of forward and reverse primers for each gene - 5 pmol per reaction. Initial DNA denaturation at 95 °C for 8 min was followed by 45 cycles performed under the regime: 95 °C – 25 sec., 58 °C – 1 min, 72 °C – 1 min; a final elongation was carried out at 72 °C for 10 min.
PCR products were analyzed in 1% agarose gel with ethidium bromide; the result was considered as positive in the case of detecting PCR product of expected length. All positive samples were sequenced using corresponding primers. Purified PCR fragments of the abovementioned genes were sequenced on an automated sequencer ABI Prism 3130 (”Applied Biosystems”, USA). The obtained sequences were compared with reference sequences of Mycoplasma sp. 1220 from GenBank (National Center for Biotechnology Information - NCBI) (respectively EU596576, HQ380795, HQ380794 and HQ438039). Alignment of nucleotide sequences was performed in ClustalW program and phylogenetic dendrograms were designed using the algorithm ”minimum-evolution” at bootstrap parameter of 1000 repetitions (MEGA 4; http://www.megasoftware.net).
Results. This study was the first research on description and genetic identification of Mycoplasma sp. 1220 in domestic geese with clinical symptoms of salpingitis, inflammation of phallus and cloaca in the territory of Russia and Ukraine upon own earlier findings on Mycoplasma sp. 1220 isolates derived from geese of Hungary.
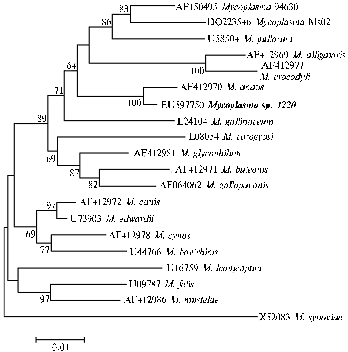
The data of sequencing and analysis of ribosomal genes 16S-rRNA, b-subunit of DNA-dependent RNA polymerase rpoB and ribosomal intergenic spacer 16S-23S rRNA in different Mycoplasma species show (29) that Mycoplasma sp. 1220 isolated in Hungary is related to a phylogenetic cluster M. synoviae, and the most close species is M. anatis (Fig. 1) described in domestic and wild ducks (12, 30). Until this time, Mycoplasma sp. 1220 had been isolated only in domestic geese (13, 14, 23) .
|
Fig. 1. Dendrogram showing phylogenetic relations of Mycoplasmasp. 1220 derived from geese in Hungary with other microorganisms in the cluster M. synoviae designed using the algorithm ”minimum-evolution” upon nucleotide sequences of 16S-rRNA gene (29). |
1. The proportion of differences in nucleotide sequences (%) of genes for 16S-rRNA and b-subunit of DNA-dependent RNA-polymerase rpoB between various species of the phylogenetic cluster Mycoplasma synoviae |
||||||||||||||||||||||||||||
Species |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
18 |
19 |
20 |
21 |
22 |
23 |
24 |
24 |
26 |
27 |
28 |
M. anatisK6193A (1) |
100 |
99,6 |
99,2 |
90,5 |
90,5 |
90,5 |
79,7 |
78,1 |
77,7 |
78,2 |
79,1 |
79,9 |
75,8 |
79,1 |
73,8 |
77,6 |
78,4 |
77,0 |
74,9 |
75,0 |
72,7 |
77,0 |
78,8 |
77,5 |
77,7 |
79,2 |
78,4 |
75,5 |
M. anatisK6193C (2) |
100 |
100 |
99,1 |
90,3 |
90,3 |
90,3 |
79,6 |
78,0 |
77,5 |
78,0 |
79,1 |
79,8 |
75,7 |
78,9 |
73,6 |
77,4 |
78,3 |
76,8 |
74,8 |
74,9 |
72,5 |
77,1 |
78,6 |
77,3 |
77,9 |
79,2 |
78,2 |
75,5 |
M. anatis 1340 (3) |
99,7 |
99,7 |
100 |
90,4 |
90,4 |
90,4 |
79,4 |
77,9 |
77,4 |
78,2 |
79,1 |
80,1 |
75,7 |
79,2 |
74,0 |
77,3 |
78,5 |
76,9 |
74,7 |
74,7 |
72,3 |
77,0 |
78,8 |
77,4 |
77,7 |
79,6 |
78,2 |
75,5 |
M. anserisalpingitis31948 (4) |
99,2 |
99,2 |
98,9 |
100 |
100 |
100 |
80,4 |
78,9 |
78,4 |
78,9 |
79,3 |
80,5 |
77,6 |
79,6 |
74,0 |
76,8 |
78,1 |
77,0 |
75,4 |
73,9 |
72,9 |
78,4 |
79,5 |
78,4 |
77,7 |
79,6 |
78,1 |
76,6 |
M. anserisalpingitis31848 (5) |
99,2 |
99,2 |
98,9 |
100 |
100 |
100 |
80,4 |
78,9 |
78,4 |
78,9 |
79,3 |
80,5 |
77,6 |
79,6 |
74,0 |
76,8 |
78,1 |
77,0 |
75,4 |
73,9 |
72,9 |
78,4 |
79,5 |
78,4 |
77,7 |
79,6 |
78,1 |
76,6 |
M. anserisalpingitis1220 (6) |
99,2 |
99,2 |
98,9 |
99,8 |
99,8 |
100 |
80,4 |
78,9 |
78,4 |
78,9 |
79,3 |
80,5 |
77,6 |
79,6 |
74,0 |
76,8 |
78,1 |
77,0 |
75,4 |
73,9 |
72,9 |
78,4 |
79,5 |
78,4 |
77,7 |
79,6 |
78,1 |
76,6 |
M. pullorum(7) |
94,9 |
94,9 |
94,6 |
94,8 |
94,8 |
94,8 |
100 |
78,6 |
76,9 |
77,0 |
77,8 |
78,7 |
75,8 |
76,5 |
75,0 |
76,8 |
77,7 |
76,6 |
76,9 |
71,2 |
73,3 |
75,6 |
77,7 |
77,8 |
78,2 |
77,2 |
79,5 |
74,8 |
M. alligatoris(8) |
94,3 |
94,3 |
94,1 |
94,3 |
94,3 |
94,4 |
92,5 |
100 |
85,2 |
77,6 |
78,4 |
78,8 |
74,9 |
77,8 |
76,5 |
76,7 |
77,1 |
77,6 |
75,0 |
74,6 |
73,5 |
76,8 |
79,1 |
78,3 |
78,3 |
77,7 |
76,1 |
76,2 |
M. crocodyli(9) |
94,1 |
94,1 |
93,9 |
94,1 |
94,1 |
94,1 |
91,7 |
97,9 |
100 |
77,0 |
76,7 |
77,4 |
74,2 |
76,7 |
75,0 |
75,0 |
75,7 |
76,2 |
74,9 |
72,8 |
72,7 |
75,8 |
78,4 |
78,1 |
76,7 |
76,8 |
78,5 |
76,2 |
M. verecundum(10) |
93,4 |
93,4 |
93,2 |
93,2 |
93,2 |
93,2 |
91,8 |
92,7 |
92,4 |
100 |
82,5 |
81,4 |
79,8 |
81,0 |
79,7 |
79,6 |
81,5 |
79,2 |
80,0 |
76,6 |
77,4 |
79,2 |
79,4 |
79,1 |
80,0 |
80,5 |
80,7 |
75,7 |
M. gallinaceum(11) |
94,6 |
94,6 |
94,4 |
94,8 |
94,8 |
94,8 |
93,4 |
92,9 |
92,5 |
93,2 |
100 |
82,3 |
80,3 |
82,2 |
76,7 |
77,3 |
80,1 |
77,7 |
78,0 |
74,3 |
75,7 |
77,5 |
81,4 |
79,5 |
82,4 |
81,8 |
82,0 |
77,8 |
M. buteonis(12) |
94,5 |
94,5 |
94,2 |
94,6 |
94,6 |
94,6 |
92,2 |
93,1 |
93,0 |
92,3 |
93,4 |
100 |
81,9 |
85,0 |
79,1 |
80,0 |
82,9 |
79,6 |
79,5 |
76,2 |
77,4 |
80,1 |
80,2 |
80,4 |
79,9 |
81,3 |
81,1 |
75,5 |
M. gallopavonis(13) |
94,7 |
94,7 |
94,4 |
95,1 |
95,1 |
95,1 |
92,3 |
93,1 |
92,6 |
92,6 |
93,9 |
96,6 |
100 |
84,6 |
76,9 |
79,4 |
79,6 |
77,3 |
77,6 |
74,6 |
74,1 |
78,3 |
77,4 |
77,2 |
77,8 |
78,8 |
78,1 |
74,3 |
M. glycophilum(14) |
95,1 |
95,1 |
94,8 |
95,3 |
95,3 |
95,3 |
93,4 |
93,1 |
92,6 |
93,9 |
94,8 |
96,3 |
96,9 |
100 |
76,9 |
78,9 |
81,2 |
78,2 |
77,8 |
76,7 |
76,0 |
78,0 |
80,4 |
78,0 |
78,4 |
81,0 |
80,4 |
76,0 |
M. corogypsi(15) |
94,7 |
94,7 |
94,4 |
94,5 |
94,5 |
94,5 |
91,9 |
92,3 |
91,9 |
92,4 |
93,8 |
94,8 |
94,1 |
95,4 |
100 |
75,6 |
78,1 |
76,5 |
75,8 |
74,3 |
74,8 |
76,7 |
77,4 |
77,2 |
77,2 |
76,3 |
76,4 |
74,7 |
M. canis(16) |
94,6 |
94,6 |
94,3 |
94,9 |
94,9 |
94,9 |
92,5 |
93,0 |
92,7 |
93,9 |
94,2 |
95,1 |
94,9 |
95,8 |
94,4 |
100 |
89,1 |
85,2 |
82,4 |
77,2 |
77,5 |
83,0 |
78,2 |
75,9 |
75,1 |
76,7 |
76,6 |
73,5 |
M. edwardii(17) |
95,3 |
95,3 |
95,1 |
95,6 |
95,6 |
95,6 |
93,3 |
93,4 |
93,1 |
94,4 |
94,5 |
95,7 |
95,1 |
96,4 |
95,1 |
98,7 |
100 |
85,1 |
83,7 |
78,0 |
78,4 |
84,4 |
78,3 |
77,2 |
77,1 |
79,8 |
78,2 |
75,6 |
M. cynos(18) |
94,4 |
94,4 |
94,1 |
94,7 |
94,7 |
94,7 |
92,2 |
92,3 |
92,1 |
94,0 |
93,6 |
94,1 |
94,6 |
95,3 |
93,5 |
97,5 |
97,5 |
100 |
82,7 |
78,7 |
78,4 |
83,0 |
79,4 |
78,0 |
77,3 |
78,8 |
77,8 |
74,1 |
M. bovirhinis(19) |
93,8 |
93,8 |
93,5 |
93,6 |
93,6 |
93,6 |
91,7 |
92,6 |
92,0 |
93,2 |
93,4 |
93,4 |
93,6 |
94,1 |
93,4 |
96,8 |
96,5 |
96,7 |
100 |
76,7 |
76,9 |
81,1 |
77,9 |
77,2 |
77,8 |
78,0 |
78,7 |
75,4 |
M. felis(20) |
93,7 |
93,7 |
93,5 |
93,5 |
93,5 |
93,5 |
90,8 |
91,7 |
91,5 |
92,6 |
92,7 |
92,9 |
93,4 |
94,5 |
93,3 |
94,6 |
94,9 |
94,9 |
94,2 |
100 |
77,9 |
79,1 |
75,6 |
75,3 |
73,6 |
74,7 |
72,5 |
72,9 |
M. mustelae(21) |
94,5 |
94,5 |
94,2 |
94,3 |
94,3 |
94,3 |
92,1 |
92,5 |
92,1 |
93,4 |
93,0 |
93,1 |
93,3 |
94,6 |
93,6 |
95,3 |
95,3 |
95,3 |
95,1 |
95,8 |
100 |
79,2 |
76,7 |
74,9 |
75,4 |
75,3 |
76,5 |
73,3 |
M. leonicaptivi(22) |
93,4 |
93,4 |
93,1 |
93,3 |
93,3 |
93,3 |
91,5 |
91,5 |
91,4 |
92,0 |
91,7 |
93,1 |
92,3 |
93,3 |
92,9 |
94,5 |
94,9 |
94,5 |
94,0 |
93,7 |
94,8 |
100 |
77,6 |
77,1 |
76,3 |
77,9 |
76,7 |
75,1 |
M. oxoniensis(23) |
94,1 |
94,1 |
93,8 |
94,1 |
94,1 |
94,1 |
92,1 |
92,7 |
92,1 |
92,0 |
92,9 |
92,4 |
93,1 |
93,8 |
92,2 |
91,8 |
92,6 |
92,5 |
91,8 |
92,7 |
92,6 |
91,6 |
100 |
90,1 |
84,1 |
82,8 |
81,1 |
78,2 |
M. cricetuli(24) |
94,1 |
94,1 |
93,8 |
94,0 |
94,0 |
94,0 |
91,8 |
92,4 |
91,8 |
91,8 |
92,4 |
92,7 |
92,8 |
93,4 |
92,2 |
91,7 |
92,4 |
92,7 |
91,6 |
92,7 |
92,7 |
91,7 |
98,8 |
100 |
84,2 |
83,3 |
81,5 |
76,0 |
M. citelli(25) |
93,4 |
93,4 |
93,1 |
93,4 |
93,4 |
93,4 |
91,5 |
92,7 |
91,9 |
92,7 |
92,7 |
92,4 |
92,7 |
93,4 |
91,9 |
92,1 |
92,6 |
92,2 |
92,5 |
92,4 |
93,0 |
91,5 |
96,3 |
96,0 |
100 |
86,0 |
83,8 |
77,3 |
M. columborale(26) |
94,7 |
94,7 |
94,4 |
94,8 |
94,8 |
94,8 |
92,8 |
92,8 |
92,7 |
93,3 |
93,5 |
94,3 |
94,6 |
94,9 |
93,2 |
93,2 |
94,1 |
93,4 |
92,4 |
93,1 |
93,5 |
92,6 |
96,9 |
96,9 |
97,0 |
100 |
85,4 |
76,4 |
M. sturni(27) |
92,5 |
92,5 |
92,2 |
92,7 |
92,7 |
92,7 |
90,4 |
91,8 |
91,4 |
92,0 |
92,7 |
93,2 |
92,9 |
93,2 |
91,5 |
92,7 |
93,2 |
92,2 |
92,1 |
91,6 |
92,1 |
90,5 |
93,8 |
93,7 |
94,3 |
95,3 |
100 |
75,8 |
M. synoviae(28) |
91,1 |
91,1 |
90,8 |
90,9 |
90,9 |
90,9 |
89,8 |
90,4 |
89,9 |
90,9 |
90,8 |
90,7 |
90,5 |
91,2 |
90,3 |
91,5 |
91,7 |
92,1 |
92,0 |
91,2 |
92,3 |
90,8 |
91,4 |
91,4 |
91,8 |
91,4 |
90,5 |
100 |
Note. Characteristics for genes 16S-rRNA and rpoB are shown correspondingly above and below the diagonal of 100 % identity to each species |
||||||||||||||||||||||||||||
A comparative analysis of nucleotide sequences of ribosomal 16S-rRNA genes and b-subunit of DNA-dependent RNA polymerase rpoB of Mycoplasma sp. 1220 (Mycoplasma anserisalpingitis) strains isolated in Hungary, typical and clinical strains of M. anatis isolated from wild ducks in the USA, as well as typical strains related to a phylogenetic cluster M. synoviae allowed to conclude that nucleotide sequences of 16S-rRNA genes of Mycoplasma sp. 1220 and M. anatis show a significant similarity and therefore can’t be used to identify the species of mycoplasma isolates derived from geese.
At the same time it was found that nucleotide differences in rpoB gene for b-subunit of DNA-dependent RNA polymerase in Hungarian isolate Mycoplasma sp. 1220 and M. anatis, as well as in other mycoplasmas belonging to the cluster M. synoviae (Table 1) are sufficient for an accurate discrimination of these species. Besides, two previously described species isolated from Chinese hamsters – M. oxoniensis and M. cricetuli – have phylogenetic closeness within the cluster M. synoviae similar to the close resemblance between Mycoplasma sp. 1220 and M. anatis (Table 1.).
The revealed possibility of identification of mycoplasma species by comparing the nucleotide sequence of rpoB gene was used in the analysis of pathological material collected in the studied geese farms of Russia and Ukraine.
In these farms there was recorded high mortality of goose embryos during incubation and reduce in egg production of she-geese. At the same time, microorganisms of genera Pasteurella, Salmonella, Neisseria, Escherichia, and M. gallisepticum and M. synoviae were not found in the studied samples, as well as antibodies to a bird flu virus and Newcastle disease in the blood serum.
Earlier in poultry farms of Hungary there was described a disease of geese associated with Mycoplasma sp. 1220 and similar clinical presentation: increased mortality of livestock, egg shell deformation, reduce in egg production to 25%, and increased embryonic mortality in the last days up to 40% (14, 26). Sick individuals manifested seroplastic peritonitis and salpingitis, and inflammation of phallus in males (10, 14). 15% of such cases were accompanied by a necrotic enteritis with fibrinous exudate (14), which, though, wasn’t a common symptom of the disease caused by Mycoplasma sp. 1220 and probably was induced by pathogenic microorganisms arisen against the background of primary mycoplasmosis. Mycoplasma sp. 1220 was isolated from pathologically altered air sucks, liver, spleen, testes, ovaries, oviduct and peritoneum of sick geese (14, 26).
Thus, the presence of Mycoplasma sp. 1220 in tested samples was assumed upon the clinical symptoms of sick geese in Russia and Ukraine which were similar to the previously described in Hungary, along with the absence of the abovementioned bacterial and viral pathogens.
Testing DNA samples isolated from the pathological material was performed using primers designed for a specific detection of Mycoplasma sp. 1220 (Table 2).
The first assumption was confirmed by PCR with four genetic markers of Mycoplasma sp. 1220 – a positive result for all the genes was found in 23 (49%) of 47 tested samples. At the same time, 15 samples of laryngeal swabs from clinically healthy geese showed negative results.
| 2. Nucleotide sequences of primers developed for amplification of gene fragments of Mycoplasma sp. 1220 isolated from samples of investigated pathological material. | |||
Primer type |
Nucleotide sequence of primer |
Target gene |
Amplicon length, bp |
Forward |
5´-CAA-ACA-TTA-GGA-GCA-AAG-AAT-TCG-3´ |
Gene for a-subunit of DNA-polymerase III (dnaE) |
800 |
Reverse |
5´-TGG-TTT-CGC-TAA-TTT-CTG-GAT-CG-3´ |
||
Forward |
5´-ACA-TTA-TTG-ATA-CAC-CAG-GGC-ACG-3´ |
G-factor of translation and elongation (fusA) |
1000 |
Reverse |
5´-GGT-TCA-AGA-GCT-TGT-GAA-ATA-ACT-GG-3´ |
||
Forward |
5´-ACT-TCA-GTC-ATG-GAG-ATC-ACT-C-3´ |
Gene for pyruvate kinase (pyk) |
850 |
Reverse |
5´-TCA-AGT-GGG-AAT-TTA-CCG-TTT-GCA-G-3´ |
||
Forward |
5´-TAA-CCA-AAT-TCC-AAT-TGT-AAA-ACT-TGG-TG-3´ |
Gene for b-subunit of RNA-polymerase (rpoB) |
532 |
Reverse |
5´-CAA-GAA-CAT-CAC-CAA-GTT-CTC-TAC-3´ |
||
Mycoplasma sp. 1220 (as well as M. anseris, M. cloacale and Acholeplasma spp.) was earlier isolated from clinically healthy geese in Germany (23). So, manifestation of its pathogenic properties in vivo can be associated with keeping conditions, varying virulence of its strains, dysbiosis in the cloaca and oviduct against other infections and/or improper zoohygienic environment. Since these mycoplasma present in a normal microflora of geese without causing visible clinical and pathological changes, pathogenesis can be induced by stressful factors that weaken the organism, such as over-compacted flocks and/or absence of water reservoir (Z. Varga and L. Stipkovits, personal report).
In addition, this infection was clinically more pronounced in ganders (inflammation of phallus and cloaca), while in she-geese its symptoms (inflammation of the oviduct and cloaca) were not always clinically manifested, so the major symptom of infection are reduced egg production and visible deformation of egg shell (projections and rough grainy surface due to calcium deposits, later – a significantly thinned shell) and embryonic mortality (possibly due to a vertical infection) (14).
|
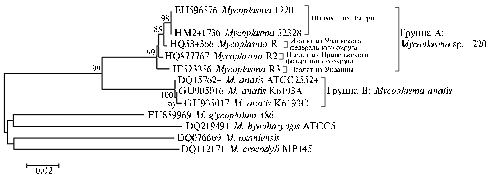
Fig. 2. Dendrogram showing phylogenetic relation of Mycoplasmasp. 1220 isolates derived from two geese farms of Russian Federation and one farm of Ukraine designed using the algorithm ”minimum-evolution” upon nucleotide sequences of rpoB gene (29). |
Sequencing of all obtained PCR fragments have demonstrated 98-99% their homology to Mycoplasma sp. 1220 sequence from GenBank isolated and identified in domestic geese in Hungary present. Figure 2 shows the results of phylogenetic analysis of nucleotide sequences of the gene for b-subunit of DNA-dependent RNA polymerase (rpoB) in Mycoplasma sp. 1220 samples derived in Russia and Hungary.
Representative sequences of Mycoplasma sp. 1220 gene for b-subunit of DNA-dependent RNA polymerase (rpoB), a-subunit of DNA polymerase III (dnaE), elongation factor (fusA), pyruvate kinase gene (pyk) of detected in geese in Russia, have been deposited in GenBank with numbers HQ534366, HQ877767, JF523356 and JF731008-JF731011.
So, this work was the first case of detection of Mycoplasma sp. 1220 in domestic geese kept in poultry farms of Russia and Ukraine. In the studied samples of biological material obtained from sick geese with clinically manifested inflammation of phallus, cloaca and oviduct there wasn’t detected Pasteurella, Salmonella, Neisseria and Escherichia, as well as Mycoplasma gallisepticum and M. synoviae, no serum antibodies to a bird flu virus and Newcastle disease. At the same time, these samples were found to carry specific amplicons of genes rpoB, dnaE, fusA, pyk of Mycoplasma sp. 1220. Sequencing of these amplicons have demonstrated 98-99% homology with the corresponding sequences of Mycoplasma sp. 1220 presented in GenBank.
REFERENCES
1. Bradbury J.M., Gordon Memorial Lecture. Poultry Mycoplasmas: Sophisticated Pathogens in Simple Guise, Br. Poult. Sci., 2005, vol. 46, pp. 125-136.2. Stipkovits L. and Kempf I., Mycoplasmoses in Poultry, Rev. Sci. Tech., 1996, vol. 15, pp. 1495-525.
3. Lysko S.B., Skhemy profilaktiki i lecheniya respiratornogo i assotsiativnogo mikoplazmoza ptits (Regimens for Prophylaxis and Treatment of Respiratory and Associative Mycoplasmosis in Poultry), Cand. Sci. Dissertation, Omsk, 2005.
4. Suntsova O.A., Usovershenstvovanie laboratornoi diagnostiki assotsiativnogo respiratornogo mikoplazmoza ptits (Improvement of Laboratory Diagnostics of Associative Respiratory Avian Mycplasmosis), Cand. Sci. Dissertation, Omsk, 2004.
5. Kleven S.H., Control of Avian Mycoplasma Infections in Commercial Poultry, Avian Dis., 2008, vol. 52, no. 3, pp. 367-374.
6. Noormohammadi A.H., Role of Phenotypic Diversity in Pathogenesis of Avian Mycoplasmosis, Avian Pathol., 2007, vol. 36, no. 6, pp. 439-444.
7. Mudrak N.S., Sozdanie i vnedrenie v promyshlennoe ptitsevodstvo sistemy kompleksnogo serologicheskogo monitoringa infektsionnykh boleznei na osnove immunofermentnogo analiza (Development and Implementation in Commercial Poultry Farming of Integrated Disease Monitoring System Based on Immunoenzyme Assay), Doctoral Sci. Dissertation, Vladimir, 2010.
8. Stipkovits L., El-Ebeedy A.A., Kisary J. and Varga L., Mycoplasma Infection of Geese. 1. Incidence of Mycoplasmas and Acholeplasmas in Geese, Avian Pathol., 1975, no. 4, pp. 35-43.
9. Kisary J., El-Ebeedy A.A. and Stipkovits L., Mycoplasma Infection of Geese. II. Studies on Pathogenicity of Mycoplasmas in Goslings and Goose and Chicken Embryos, Avian Pathol., 1976, no. 5, pp. 15-20.
10. Stipkovits L., Bove J.M., Rousselot M., Larrue P., Labat M. and Vuillaume A.., Studies on Mycoplasma Infection of Laying Geese, Avian Pathol., 1985, vol. 14, pp. 57-68.
11. Benoina D., Tadina T. and Dorrer D., Natural Infection of Geese with Mycoplasma gallisepticum and Mycoplasma synoviae and Egg Transmission of the Mycoplasmas, Avian Pathol., 1988, vol. 17, no. 4, pp. 925-928.
12. Bencina D., Dorrer D. and Tadina T., Mycoplasma SpeciesIsolated from Six Avian Species, Avian Pathol., 1987, vol. 16, no. 4, pp. 653-664.
13. Varga Z., Stipkovits L., Dobos-Kovács M. and Sántha M., Investigation of Goose Mycoplasmas, Arch. Exp. Veterinarmed, 1986, vol. 40, no. 1, pp. 105-108.
14. Dobos-Kovacs M., Varga Z., Czifra G. and Stipkovits L., Salpingitis in Geese Associated with Mycoplasma sp. Strain 1220. Avian Pathol., 2009, vol. 38, pp. 239-243.
15. Behr K.P., Hinz K.H. and Rottmann S., Phallus-Inflammation of Ganders: Clinical Observations and Comparative Bacteriological Examinations of Healthy and Altered Organs, Zentralbl. Veterinarmed. B, 1990, vol. 37, no. 10, pp.10): 774-776.
16. Nunoya T., Kanai K., Yagihashi T., Hoshi S., Shibuya K. and Tajima M., Natural Case of Salpingitis Apparently Caused by Mycoplasma gallisepticum in Chickens, Avian Pathol., 1997, vol. 26, pp. 391-398.
17. Degernes L.A., Waterfowl Toxicology: a Review, Vet. Clin. North Am. Exot. Anim. Pract., 2008, no. 11, pp. 283-300.
18. Palyusik M. and Koplik-Kovacs E., Effect on Laying Geese of Feeds Containing the Fusariotoxins T2 and F2, Acta Vet. Acad. Sci. Hung., 1975, vol. 25, no. 4, pp. 363-368.
19. Pataky M., An Infectious Inflammatory Disease of Cloaca and Penis in Geese (Goose Gonorrhoea). II. Properties of the Causal Agent and Infection Experiments, Acta Vet. Acad. Sci. Hung., 1974, vol. 24, pp. 335-359.
20. Szep I., Pataky M. and Nagy G., An Infectious Inflammatory Disease of Cloaca and Penis in Geese (Goose Gonorrhoea). I. Epizootological, Clinical, Pathological Observations and Control, Acta Vet. Acad. Sci. Hung., 1974, vol. 24, pp. 347-354.
21. Nalivayko L.I., Biologicheskie svoistva vozbuditelya neisserioza gusei i diagnostika bolezni (Biological Properties of Casual Agent of Neisseriosis of Geese and Its Diagnostics), Borki, 1984.
22. Beemer A.M., Kuttin E.S. and Katz Z.., Epidemic Venereal Disease due to Candida Albicans in Geese in Israel, Avian Dis., 1973, vol. 17, pp. 639-649.
23. Hinz K.H., Pfutzner H. and Behr K.P., Isolation of Mycoplasmas from Clinically Healthy Adult Breeding Geese in Germany, Zentralbl Veterinarmed, 1994, vol. B 41, pp. 145-147.
24. Stipkovits L., The Pathogenicity of Avian Mycoplasmas, Zentralbl. Bakteriol. Orig. A, 1979, vol. 245, no. 1-2, pp. 171-183.
25. Stipkovits L., Glavits R., Ivanics E. and Szabo E., Additional Data on Mycoplasma Disease of Goslings, Avian Pathol., 1993, vol. 22, pp. 171-176.
26. Stipkovits L., Varga Z., Czifra G. and Dobos-Kovacs M., Occurrence of Mycoplasmas in Geese Affected with Inflammation of the Cloaca and Phallus, Avian Pathol., 1986, vol. 15, pp. 289-299.
27. Stipkovits L., Varga Z., Glavits R., Ratz F. and Molnar E., Pathological and Immunological Studies on Goose Embryos and One-Day-Old Goslings Experimentally Infected with a Mycoplasma Strain of Goose Origin, Avian Pathol., 1987, vol. 16, pp. 453-468.
28. Stipkovits L., Varga Z., Dobos-Kovacs M. and Santha M., Biochemical and Serological Examination of Some Mycoplasma Strains of Goose Origin, Acta Vet. Hung., 1984, vol. 32, no. 3-4, pp. 117-125.
29. Volokhov D.V., Simonyan V., Davidson M.K. and Chizhikov V.E., RNA Polymerase Beta Subunit (rpoB) Gene and the 16S-23S rRNA Intergenic Transcribed Spacer Region (ITS) as Complementary Molecular Markers in Addition to the 16S rRNA Gene for Phylogenetic Analysis and Identification of the Species of the Family Mycoplasmataceae, Mol. Phylogenet. Evol., 2012, vol. 62, no. 1, pp. 515-528.
30. Samuel M.D., Goldberg D.R., Thomas C.B., Sharp P., Robb J.R., Krapu G.L., Nersessian B.N., Kenow K.P., Korschgen C.E., Chipley W.H. and Conroy M.J., Exposure of Wild Waterfowl to Mycoplasma anatis, J. Wildl. Dis., 1996, vol. 32, no. 2, pp. 331-337.
31. Czifra G., Varga Z., Dobos-Kovacs M. and Stipkovits L., Medication of Inflammation of the Phallus in Geese, Acta Vet. Hung., 1986, vol. 34, pp. 211-223.
32. OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 2011, http://www.oie.int/international-standard-setting/terrestrial-manual/access-online/.
33. Sprygin A.V., Andreychuk D.B., Kolotilov A.N., Volkov M.S., Runina I.A., Mudrak N.S., Borisov A.V., Irza V.N., Drygin V.V. and Perevozchikova N.A., Development of a Duplex Real-Time TaqMan PCR Assay with an Internal Control for the Detection of Mycoplasma gallisepticum and Mycoplasma synoviae in Clinical Samples from Commercial and Backyard Poultry, Avian Pathol, vol. 39, pp. 99-109.
1Federal Center for Animal Health Protection, Vladimir 600991, Russia, |
Received June 6, 2011
|