doi: 10.15389/agrobiology.2012.2.64eng
УДК 636.1:636.082:591.463.1:576.08
RESISTANCE OF STALLION’S SPERM TO FREEZING DUE TO INTRODUCTION OF SKQ1 ANTIOXIDANT TO THE MEDIUM
V.A. Naumenkova1, E.E. Bragina2, E.V. Nikitkina3
The cryoresistance of stallion’s sperm was studied during deep cooling and freezing after introduction to the medium of antioxidant SkQ1 preparation. It was established, that SkQ1 introduction rises the resistance of stallion’s sperm to freezing due to improvement of safety of acrosomes, mitochondrions and cellular membranes, and thus, lead to the increase of semen fertilizing capacity.
Keywords: sperm, freezing, medium, SkQ1 component, fertility.
Cryopreservation of cells is widely used in modern reproductive technologies as well as in biodiversity conservation (1, 2). The basic technique used at cryopreservation of semen for artificial insemination is sperm dilution. Improvement of a dilution medium is still an urgent task, since the post-thaw activity of sperm cells reduces by 30-40% of the initial value, which results in reduced fertilizing capacity.
Native semen of stallions, as well as boar sperm, is significantly diluted with secretions of genital glands and is subject to oxidation reactions undesirable at storage. Substances inhibiting the oxidation of sperm of these species are being sought for (3). Antioxidants reducing intense oxidation of a fresh ejaculate are of particular interest. Introduction of antioxidants to semen provides hypoxic effects: these substances decrease the content of activated oxygen in the media, inhibit lipid peroxidation, reduce accumulation of toxic peroxides and stabilize contents of active groups of proteins (3-7). The new generation of such products is represented by a target mitochondrial antioxidant SkQ1 structurally close to coenzyme Q10 (ubiquinone) and related to “Skulachev ions”. Biological activity of these ions is the result of their ability to accumulation in mitochondria due to a membrane potential without extra energy costs for transport (8).
Synthetic antioxidants SkQ1 have an important ability to self-restoration in mitochondrial respiratory chain and resulting prolonged multiple effects distinct from classic antioxidants whose activity is immediately lost after the interaction with free radical. SkQ1 effectively protects mitochondrial membranes from oxidation by free radicals (8).
The purpose of this work was an attempt to improve the cryopreservation technique used at deep cooling and freezing stallion sperm by introducing the antioxidant SkQ1 in sperm dilution medium along with evaluation of fertilizing capacity of the sperm treated with SkQ1.
Technique. The experiments were performed in 2006-2010 using the sperm of eight stallions of different breeds kept on experimental and sports stable (All-Russia Research and Development Institute of Horse Breeding). A semen was obtained using an artificial vagina (9). Each ejaculate was divided into 10 equal parts, one was diluted with lactose-chelate-citrate-egg yolk (LCCY) medium (control), other parts - with LCCY medium with SkQ1 (0,0010; 0,0020; 0,0025; 0,0030; 0,0040; 0,0050; 0,0060; 0,0080 and 0,0110 uM) (test) to determine an optimal concentration providing cryoprotective effect. The sperm was frozen in nitrogen vapor (9) and then transferred into liquid nitrogen. The samples were thawed in a water bath at 40 °C not earlier than 1 day after freezing.
Semen quality was assessed visually using the light microscope from sperm motility before and after freezing. Post-thaw sperm survival was determined at 2-4°C from the time of maintaining motility. Sperm ultrastructure was investigated in samples obtained from two stallions (Narzan and Tagay) – freshly obtained (undiluted) and after freezing-thawing of a sample diluted with LCCY medium with an optimum content of SkQ1 (test) and without it (control) . The samples were diluted 1:10 with NaCl isotonic solution and a fixative was added – 2,5% glutaraldehyde solution (“Ted Pella Inc.”, USA) prepared in 0,1 M cacodylate buffer (pH 7,2) (“Sigma”, USA), then centrifuged for 15 min at 1000 rpm, a supernatant was removed, a sediment was fixed in the same fixative, finally treated with 1% osmic acid solution (“Serva”, Germany) and embedded in Epon resin (“Fluka”, Germany ). Ultrathin sections were obtained on an ultramicrotome Reichert UltraSut III (Austria), finally stained with aqueous solution of uranyl acetate and lead citrate (“Serva”, Germany); the ultrastructure of acrosomes and mitochondria was examined in an electron microscope Hitachi700 (Japan). Permeability of cell membranes was assessed from the proportion (%) of cells stained with a fluorescent tag ethidium bromide according to the recommended procedure (10).
Fertilizing capacity of sperm was evaluated in ejaculate obtained from one stallion (Malysh), which was divided into two parts: one – diluted with LCCY medium supplemented with an optimum content of SkQ1, another - with the same medium without SkQ1. Pre-freezing procedures, freezing and thawing of the sperm was performed as described above. Mares (7) were divided into test and control groups (4 and 3 individuals, resp.). The first group was inseminated with the sperm frozen and thawed in the dilution medium with SkQ1, the second – with the same medium not containing SkQ1. The efficiency of insemination was accounted upon the results of washing out embryos on the 8th day after ovulation.
The data were statistically treated by variation statistics according to N.A. Plokhinsky (11).
Results. A preliminary experiment had revealed optimal cryoprotective concentrations of SkQ1 – 0,0010-0,0030 uM (Table 1). At these doses the post-thaw period of recording motile spermatozoa (sperm survival) increased in a test sample by 6 h compared with control (P> 0,95). Subsequent experiments were performed using 0,0030 uM SkQ1 as the optimal concentration providing the best post-thaw sperm survival relative to control.
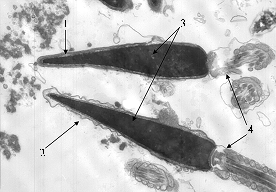
Freezing-thawing of stallion sperm most strongly affected the ultrastructure of acrosomes (Fig. 1). Intact acrosome is a flat tank with a matrix of medium electron density, closely adjacent to the nucleus and covering 2/3 frontal part of the head. After freezing-thawing, there was accounted a greater number of cells with damaged acrosome – degraded (a result of acrosomal reaction – acrosome destruction with formation of membrane vesicles and release of the contents) or “empty” (with electron-transparent matrix due to the loss of internal electron-dense content) (12). Along with it, cryopreservation increases the rate of sperm cells with mitochondria containing the matrix of abnormal electron density (12, 13).
| 1. Motility and survival of stallion sperm subject to freezing-thawing in lactose-chelate-citrate-yolk medium supplemented with different contents of antioxidant SkQ1 | |||
SkQ1, uM |
Motility, grades |
Survival at 0 °С, h |
|
before freezing |
after thawing |
||
0 |
5,0±0,20 |
2,5±0,10 |
120±10 |
0,0010 |
5,0±0,35 |
2,5±0,15 |
126±12 |
0,0020 |
5,0±0,30 |
2,5±0,20 |
126±13 |
0,0025 |
5,0±0,27 |
2,5±0,20 |
126±13 |
0,0030 |
5,0±0,30 |
2,5±0,20 |
126±14 |
0,0040 |
5,0±0,25 |
2,5±0,15 |
120±13 |
0,0050 |
5,0±0,30 |
2,0±0,20 |
108±13 |
0,0060 |
5,0±0,20 |
2,0±0,10 |
96±11 |
0,0080 |
4,5±0,20 |
1,8±0,10 |
92±10 |
0,0110 |
4,0±0,20 |
1,8±0,10 |
84±8 |
|
Fig. 1. Electron micrograph of stallion spermatozoa heads after freezing-thawing in lactose-chelate-citrate-yolk medium: 1 – intact (normal) acrosome; 2 – acrosome with electron-transparent matrix, 3 – chromatin, 4 – mid (connecting piece). Denotation: |
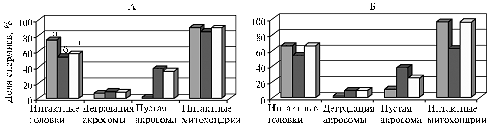
A quantitative ultrastructural analysis was performed on sperm from two stallions (Narzan and Tagay): in native semen, in control (LCCY-diluted sperm after freezing-thawing) and in experiment (LCCY-diluted sperm with 0,0030 uM SQ1 after freezing-thawing). A conventional spermiological analysis has shown a good quality of Narzan stallion’s sperm (Fig. 2), but after freezing-thawing the proportion of intact heads (i.e. having the nucleus of a correct shape, condensed chromatin and normal acrosome) decreased from the initial 75% in both control (up to 53%) and in test sample (up to 57%). The proportion of sperms with degraded acrosome didn’t depend on freezing-thawing (in the native semen, control and test – 7, 9 and 8%, resp.). The count of sperm with an electron-transparent “empty” acrosome increased from 2% (native semen) to 38 (control) and 35% (test). Cryopreservation practically didn’t affect the ultrastructure of mitochondria: the percentage of sperm with normal mitochondria in native samples, control and test – 91, 86 and 90%, resp.
The native sperm of Tagay stallion had low motility, however, SkQ1 added to the dilution medium caused a much greater influence on preservation of its ultrastructure: sperm count with intact heads in both native semen (before freezing) and after freezing-thawing in the presence SkQ1 equaled 66%.
|
|
Fig. 2. Preservation of ultrastructure of sperm obtained from stallions Narzan (A) and Tagay (B) (respectively, with high and low sperm motility upon the results of conventional spermiological investigation) before freezing (a) and after freezing-thawing in lactose-chelate-citrate-yolk medium without antioxidant SkQ1 (b) or with SkQ1 (0,0030 uM) (c). |
|
After the standard freezing and thawing (control) there was accounted only 55% sperms with intact head. SkQ1 didn’t affect the proportion of sperms with degraded acrosome: in both control and test samples it increased from 2 to 9% after freezing-thawing. At the same time, SkQ1 provided a protective effect by increasing the integrity of acrosome and mitochondrial matrix. Thus, the proportion of sperms with “empty” acrosome before freezing was 11%, after freezing and thawing – 38% in control and 25% in a sample containing SkQ1. The proportion of sperms with mitochondria of normal morphology was 96% before freezing, while after freezing and thawing it decreased to 61% in control and no differences from the native semen was recorded in the test sample diluted with a medium containing SkQ1.
| 2. Viability characteristics of stallion sperm after freezing-thawing in lactose-chelate-citrate-yolk medium supplemented with antioxidant SkQ1 | |||
Variant |
Mobility, grade |
Survival at 0 °С, h |
Permeability of cell membrane against ethidium bromide, % |
Test (SkQ1, 0,0030 uM) |
2,2±0,14 |
94±7,6 |
52,0±3,20 |
Control |
2,0±0,12 |
86±5,8 |
64,0±3,90 |
Testing the permeability of cell membrane against ethidium bromide in the presence of SkQ1 (Table 2) has shown 12,0% decrease in number of cells stained with this intercalating agent (i.e. the proportion of dead sperms with impaired membrane permeability) along with better sperm motility and survival (respectively, 9,1 and 8,5% higher) compared with control.
Fertilizing capacity of sperm was assessed using the semen from Malysh stallion completely satisfying the adopted requirements: sperm count in the ejaculate - not less than 2 x 108/ml, motility - at least 5 grades, and survival - not less than 96 hours. In mares inseminated with this sperm fertilization occurred in the test group in 3 out of 4 mares, whereas in the control group - only in 1 of 3 mares. The observed increase in fertilizing capacity of the sperm diluted with the medium containing SkQ1 after freezing and thawing can be explained by better preservation of spermatozoa ultrastructure.
Thus, the target mitochondrial antioxidant SkQ1 added to cryoprotective medium promotes better preservation of cell membranes, mitochondria and acrosomes in stallion sperm and thereby increases its resistance to freezing. Sperm cells treated with SkQ1 retain the capacity to normal fertilization, moreover, their fertilizing capacity increases. These findings suggest using SkQ1 as a cryoprotectant during freezing sperm.
REFERENCES
1. Sviridov B.E., Shapiev I.Sh. and Silin Yu.V., Cryoconservation and the Use of Blastodermal Hen Cells for the Creation of Chimeras, S.-kh. biol., 2009, no. 2, pp. 62-64.
2. Bagirov V.A., Gladyr’ E.A., Ernst L.K., Klenovitsky P.M., Zinov’eva N.A. and Nasibov Sh.N., Preservation and Efficient Use of Genetic Resources of Yak (Bos mutus), S.-kh. biol., 2009, no. 2, pp. 37-42.
3. Narizhny A.G., Action of Synthetic Antioxidants, Svinovodstvo, 1978, no. 1, pp. 24-25.
4. Prokoptsev V., Rustenov A. and Moroz L., Unithiol Effect on Boar Sperm, S.-kh. biol., 1974, vol. 9, no. 4, pp. 581-584.
5. Korban N., Moroz L. and Shapiev I., The Method of Freezing Boar Sperm, Svinovodstvo, 1977, no. 12, p. 29.
6. Nauk V.A. and Gus’kova A.M., Lipid Peroxidation at Freezing the Sperm of Bulls-Sires and Boars, Dokl. VASKhNIL, 1983, no. 2, pp. 27-29.
7. Golyshev N.A., Improving the Technique of Freezing Boar Semen, Zhyvotnovodstvo, 1985, pp. 49-51.
8. Skulachev V.P., An Attempt of Biochemists to Attack the Problem of Aging. “Megaproject” on Penetrating Ions. First Results and Prospects (Review), Biokhimiya, 2007, vol. 72, no. 12, pp. 1572-1586.
9. Rekomendatsii po zamorazhivaniyu i dlitel’nomu khraneniyu v zhidkom azote spermy zherebtsov-proizvoditelei (Recommendations on Freezing and Long-Term Storage in Liquid Nitrogen of Stallion Sperm), Moscow, 1978.
10. Aeschbacher M., Reinhardt C.A. and Zbinden G., A Rapid Cell Membrane Permeability Test using Fluorescent Dyes and Flow Cytometry, Cell Biol. Toxicol., 1986, no. 2, pp. 247-255.
11. Plokhinsky N.A., Algorithms of Biometrics, Gnedenko B.V., Ed., Moscow, 1980.
12. Alvarenga M.A., Landim-Alvarenga F.C., Moreira R.M. and Cezarino M.M., Acrosomal Ultrastructure of Stallion Spermatozoa Cryopreserved with Ethylene Glycol using Two Packaging Systems, Equine Veter. J., 2000, vol. 32, no. 6, pp. 541-545.
13. Leonardo F.C., Evaluation of Stallion Sperm Morphology, Clin. Techn. Equine Practice, 2007, vol. 6, no. 4, pp. 249-264.
1All-Russia Research and Development Institute of Horse Breeding, RAAS, Divovo 391105, Rybnovsky district, Ryazan province, Russia, |
Received July 7, 2011
|