doi: 10.15389/agrobiology.2012.2.113eng
УДК 636/639:614.31
METHODS OF SANITARY SURVEILLANCE OF LIVESTOCK PRODUCTION. V. IMMUNE-ENZYME ANALYSIS OF SULFADIMIDINE
M.A. Burkin1, G.P. Kononenko2, A.A. Burkin2
Conjugates of sulfadimidine with albumin and gelatin, synthesized by azocoupling reaction were used for induction of rabbit antibodies and optimization of immune-enzyme analysis. The antibodies to conjugate with bovine serum albumin have a high specificity and do not interact with other sulfanilamides. The indirect solid phase competitive immune-enzyme analysis on the basis of obtained antibodies and immobilized conjugate with egg albumin permits to have the sensitivity of determination of sulfadimidine of 0.2 ng/ml. The possibility of application of this assay for control of residual content of sulfadimidine in milk, meat, eggs and the norms of its addition to food is discussed.
Keywords: sulfadimidine, immunoassay, feeds, milk, meat, eggs.
Sulfonamides were the first antimicrobial agents introduced into a common veterinary practice. Later, their use decreased to some extent due to an advent of antibiotics and fluoroquinolones in recent years, but sulfonamides still retain their value (1). Sulfonamides were the basis for development of various combined medications highly efficient against infectious diseases caused by microorganisms sensitive to them, particularly Coccidiosis in poultry (2).
The most known sulfonamide is 4-amino-N-(4,6-dimethyl-2-pyrimidinyl)benzene sulfonamide, whose empirical designation is sulfadimidine (SD); its synonyms are sulfadimezine - in domestic literature and sulfamethazine - abroad:
 .
.
In the 1980ies, an enzyme-linked immunoassay (ELISA) was proposed for determination of SD in feedstuffs and livestock products along with chromatographic techniques (3-5; 6-8). In current analytical practice of foreign countries a control of SD presence in feeds and its residues in milk and meat is performed using immunoensyme test systems of various forms – from rapid assessment tests (ELISA-card, “Environmental Diagnostics, Inc.”, USA; ELISA-tube test, “Idetek, Inc.”, USA; ELISA-cap, “IDEXX Corporation”, USA) to complete analytic schemes of direct or indirect type (ELISA-wells, “SmithKline Animal Health Products”, USA; “Neogen Corporation”, USA; “R-Biopharm GmbH”, Germany). However, in Russia, there haven’t been conducted any researches aimed at establishing ELISA-based technique for selective determination of SD.
The purpose of this research was obtaining of immunoreagents highly specific to sulfadimidine (SD) and their use in indirect ELISA for detection of SD in livestock products and feedstuffs.
Technique. The work was performed using sulfamethazine (“Sigma”, USA), sodium nitrite, and organic solvents (“Fluka”, Germany), bovine serum albumin (BSA), rabbit serum albumin (RSA), egg albumin (EA), gelatin (GEL), and pharmaceuticals: sulfadimezine, sulfasalazine, ftalazol, sulgin, sulfalen, sulfadimethoxine, sulfacyl, etazol, norsulfazol, streptocide of domestic production. Antispecific enzyme conjugate was prepared from horseradish peroxidase (EC 1.11.1.7) (“Sigma”, USA) and donkey antiserum to rabbit immunoglobulin of domestic production according to manufacturers’ descriptions (9). ELISA was performed on high-binding polystyrene plates (# 9018, “Costar”, USA) using AKI-Ts-01 photometer (Russia). UV spectra were recorded on Hitachi-557 device (“Hitachi”, Japan).
Tablets of sulfonamides were grinded in a mortar and 20 ml acetone was added; after shaking the mixture was left for 14 hours and then filtered. The solution was evaporated and dry residues were weighed. To evaluate identity of the obtained substances 2 ul each solution in methanol with a concentration of 1 mg/ml was applied on plates silufol-UV 254. After chromatographic separation in the mobile phase (chloroform : methanol, 9:1) the plates were examined under UV light and treated with iodine vapor, 20% alcoholic solution of sulfuric acid and 0,3% bromophenol blue alcoholic solution. Then the weighted portions were used to prepare solutions of the same substances in 0,1 N aqueous solution of HCl and their UV spectra were recorded; the values of molar extinction for long-wave absorption maxima were calculated and compared with reference literature data (10).
Protein conjugates of SD were synthesized through azo-coupling (11). Diazonium salt was derived from equimolar quantities of SD, sodium nitrite and hydrochloric acid; then its 50-fold molar excess was added it to BSA, RSA, EA and GEL with a concentration of 10 mg/ml in 0,05 M carbonate-bicarbonate buffer (pH 9, 0-9,5). Brown-orange colored reaction mixtures were left for 2 hours at 0 °C and then dialyzed for 2 days against three changes of 1000-fold volume of 0,5% sodium chloride solution. The dialysates were added with an equal volume of glycerol and stored at -10...- 15 °C. Procedures of immunization of rabbits, testing antisera and ELISA were carried out as described previously (12).
SD residues were determined by the own developed method in following objects: 20 samples of whole cow milk, 7 samples of milk powder, samples of chicken meat and eggs obtained from a commercial network market of Moscow.
Results. A thin-layer liquid chromatography showed the absence of any impurities in the sulfadimidine derived from a pharmaceutical. Its chromatographic mobility and detection on plates after treating with 1% potassium permanganate solution in 0,25 M sulfuric acid wasn’t distinct from those of sulfamethazine produced by “Sigma” (USA). Another evidence of its high purity was good reproducibility of spectrophotometrically determined concentration in two types of gravimetrically prepared water solutions (Table 1).
| 1. Comparative spectrophotometric analysis of solutions of sulfadimidine (SD) derived from a domestically produced pharmaceutical | ||
Parameter |
Dissolvent |
|
0,1 N HCl |
0,1 N NaOH |
|
Characteristics of UV-spectrum of SD (10): |
|
|
lmax., nm |
243 |
242; 258 |
specific adsorption |
541 |
760; 783 |
Concentration of SD, ug/ml: |
|
|
by weight |
11,0 |
11,0; 11,0 |
by UV-adsorption |
10,7 (e = 15056) |
10,4 (e = 21151); 10,5 (e = 21791) |
Note. e — molar extinction. |
||
Testing the other sulfonamides obtained from the pharmaceuticals, it was found that concentrations of their solutions in 0,1 N HCl determined gravimetrically almost completely coincided with spectrophotometrically found ones as well. In a further analysis, initial solutions of these substances were diluted with acetonitrile. In UV spectrum of SD acetonitrile solution a long-wavelength absorption maximum was shifted towards higher values – to l= 268 nm, while e value remained almost unchanged and equaled 21685 ± 845 (n = 5). These parameters were necessary to clarify concentrations of SD in calibration solutions during the analysis.
SD derived from the pharmaceutical tablets was used as a hapten in conjugation with proteins. During azo-coupling, rapid coloration of the reaction mixtures to brownish-orange indicated attachment of the substance. Spectrophotometric analysis of products of SD reaction with albumins and GEL revealed a uniform sharp elevation of intensity of the absorption peak in the region from 250 to 300 nm. Since the long-wavelength maximum of SD is located near the absorption peak of protein carriers (l = 280 nm), it also confirmed binding of the hapten to macromolecules.
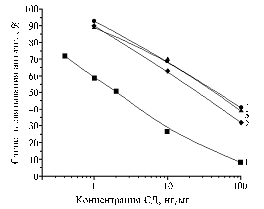
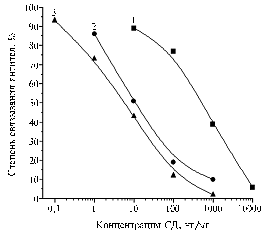 |
Fig. 1. Degree of binding of antibodies to BSA-SD(50) derived at the 1st-3rd blood samplings (1-3) with a solid-phase antigen BSA-SD(50) at the concentration of 0,05 ug/ml in presence of SD in PBST: BSA, SD, PBST – respectively, bovine serum albumin, sulfadimidine, phosphate-buffered saline with Twin 20. Denotations: |
BSA-SD(50) conjugate already after the 2nd injection (at the 1st blood sampling) provided obtaining antibodies with a working titer 1:5000 allowing determination of SD in solutions with its content up to 10 ng/ml. Continuation of the immunization procedure contributed to increase of the working titer up to 1:10 000 followed by a gradual decline to 1:5000 and then to 1:2500. After the third injection of the immunogen (2nd serum), test sensitivity increased by an order, and after the 4th injection (3rd serum) the analytical signal started a trend to a stable dependence from SD concentration (Fig. 1). After the 5th immunizing injection (4th serum) the working titles worsened up to 1:2500 as well as the quality of competitive interaction.
Among the three immobilized SD conjugates with different protein carriers only BSA-SD(50), and EA-SD(50) provided a linear analytical signal in the concentration range of SD 0,1 – 100 ng/ml. The third conjugate – GEL-SD(50) was immunoreactive but couldn’t ensure normal conditions for a competitive interaction (Table 2); at the same time, during the analysis with immobilized EA-SD(50), the increase of SD concentration from 0,1 to 100 ng/ml was associated with weakening inhibition of the reaction (binding degree reduced from 93 to 12%).
| 2. Degree of binding (%) of antibodies to BSA-SD(50) in serum from the 3rd blood sampling with different immobilized antigens in presence of SD (ELISA). | ||||
Immobilized antigen |
SD, ng/ml |
|||
100 |
10 |
1 |
0,1 |
|
BSA-SD(50) |
13 |
44 |
74 |
90 |
RSA-SD(50) |
16 |
43 |
72 |
89 |
EA-SD(50) |
12 |
38 |
66 |
93 |
GEL-SD(50) |
25 |
61 |
80 |
94 |
Note. SD, BSA, RSA, EA, GEL – respectively, sulfadimidine, bovine serum albumin, rabbit serum albumin, egg albumin, gelatin. |
||||
Cross-interaction of the obtained antibodies was evaluated in a series of SD analogs - sulfasalazine, ftalazol, sulgin, sulfalen, sulfadimethoxine, sulphacyl, etazol, norsulfazol and streptocide. The closest structural analog of SD - sulfadimethoxine - at concentrations of 100 and 1000 ng/ml showed, respectively, 87 and 67% binding, the other analogs – over 90%. Quite so high specificity was intended for antibodies aimed at discriminative detection of SD in the presence of other sulfonamides, and it was predicted from the hapten orientation in the immunogen.
|
Fig. 2. ELISA calibration charts of SD with antiserum to BSA-SD(50) from the 3rd blood sampling and a solid-phase antigen EA-SD(50) in milk at 3-fold dilution with PBST (2), in water-acetonitrile extract of chicken meat (3) and eggs (4) at 10-fold dilution with PBST: BSA, EA, SD, PBST – respectively, bovine serum albumin, egg albumin, sulfadimidine, phosphate-buffered saline with Twin 20. Denotations: |
The lower limit of detection of SD in a buffered solution considering triple average standard deviation amounted to 0,2 ng/ml (Fig. 2). A comparison of calibration curves designed under intermediate precision daily or every 1-2 days, showed the degree (%) of antibody binding (n = 10) with a relative standard deviation not exceeding 5%, i.e. satisfied the test system maintaining its functional stability under normal fluctuations of laboratory conditions.
The previously described use of indirect ELISA for detection of antibiotics in milk with its pre-dilution, as well as in water-acetonitrile extracts of feed, meat, and eggs (12) were found to be acceptable for SD as well. In milk diluted 3 times the developed test system allowed detection of SD up to 0,4 ng/ml (with inhibition of antibody binding 72%) (Fig. 2) and, therefore, provided the determination of SD residues in the products up to 1,2 ug/kg.
Testing fresh, pasteurized and sterilized milk sampled at different times, in 16 of the 20 samples the binding of antibodies amounted to 90% or more, but in 4 samples it was below 70%, which corresponded to SD contents of 10, 19, 84 and 119 ug/kg. In two of these positive samples SD content significantly exceeded MPC (25 ug/kg) (13). In 2 of the 7 tested samples of milk powder there was also found suprathreshold contents of SD - 72 and 78 ug/kg. These facts suggest the need for systematic monitoring of milk in respect to this indicator of its sanitary quality.
Calibration curve for SD in the variant with 10% aqueous acetonitrile added to a buffer (Fig. 2) demonstrate the opportunity of testing feedstuffs using extraction with aqueous acetonitrile providing the limit of detection 10 ug/kg.
Sample preparation by drying (12) can be used as simple and convenient approach in testing the safety of meat and eggs. According to the authors’ data (Fig. 2), the extractives don’t deteriorate performance of the test system. In this case, the minimum measured SD content was 2 ug/kg, whereas actual state regulations permit the presence of residual SD in meat and offal (liver, kidney) of cattle, sheep, pigs and poultry in the content of 100 ug/kg (13).
Thus, the authors have developed a highly specific technique of indirect competitive ELISA suitable for the control of residual sulfadimidine content in all types of livestock products and compliance of regulated dosage of medications containing this sulfanilamide in diets of farm animals and poultry.
REFERENCES
1. Klenova I.F. and Yaremenko N.A., Veterinarnye preparaty v Rossii. Spravochnik (Veterinary Medications in Russia: Reference Book), Moscow, 2001.
2. Spravochnik veterinarnykh preparatov (Reference Book of Veterinary Medications), Pankovets E.A., Ed., Minsk, 1996.
3. Allred M.C. and Dunmire D.L., High Performance Liquid Chromatography Determination of Sulfamethazine at Low Levels in Nonmedicated Swine Feeds, J. Chromatogr. Sci., 1978, vol. 16, no. 11, pp. 533-537.
4. Munns R.K. and Roybal J.E., Rapid Gas-Liquid Chromatographic Method for Determination of Sulfamethazine in Swine Feed, J. Assoc. Off. Anal. Chem., 1982, vol. 65, no. 5, pp. 1048-1053.
5. Houglum J.E., Larson R.D. and Neal R.M., Liquid Chromatographic Determination of Sulfamethazine in Feeds, J. Assoc. Off. Anal. Chem., 1988, vol. 71, no. 5, pp. 1054-1056.
6. McCaughey W.J., Elliott C.T. and Crooks S.R., Determination of Sulphadimidine in Animal Feedstuffs by an Enzyme-Linked Immunoassay, Food Addit. Contam., 1990, vol. 7, no. 2, pp. 259-264.
7. Dixon-Holland D.E. and Katz S.E., Competitive Direct Enzyme-Linked Immunosorbent Assay for Detection of Sulfamethazine Residues in Swine Urine and Muscle Tissue, J. Assoc. Off. Anal. Chem., 1988, vol. 71, no. 6, pp. 1137-1140.
8. Dixon-Holland D.E. and Katz S.E., Direct Competitive Enzyme-Linked Immunosorbent Assay for Sulfamethazine Residues in Milk, J. Assoc. Off. Anal. Chem., 1989, vol. 72, no. 3, pp. 447-450.
9. Nakane P.K. and Kawaoi A., Peroxidase-Labeled Antibody. A New Method of Conjugation, J. Histochem. Cytochem., 1974, vol. 22, no. 2, pp. 1084-1091.
10. Moffat A.C., Jackson J.V., Moss M.S. and Widdop B., Clarke’s Isolation and Identification of Drugs, London, 1986.
11. Hermanson G.T., Bioconjugate Techniques, San Diego-N.Y.-Boston-London-Sydney-Tokyo-Toronto, 1996, pp. 446-449.
12. Burkin A.A., Kononenko G.P. and Burkin M.A., Methods of Sanitary Control of Livestock Products. I. Enzymoimmunoassay (ELISA) of Tetracyclines, S.-kh. biol., 2010, no. 4, pp. 110-117.
13. Hygienic Requirements for Safety and Nutrition Value of Food Products. Food Raw Material and Food Products. Sanitary and Epidemiological Rules and Regulations (SanPiN) 2.3.2.1078-01, Moscow, 2002.
1I.I. Mechnikov All-Russian Research and Development Institute for Vaccines and Sera, RAMS, Moscow 105064, Russia, |
Received May 26, 2011
|