УДК 636.594:575.113:577.175.322:57.086.864
BIOLOGICAL AND PRODUCTIVE PROPERTIES OF QUAILS TRANSGENIC ON BOVINE SOMATOTROPIN GENE
L.G. Korshunova
The practical useful determinants in quails of the Estonian breed, transgenic on bovine somatotropin gene, were studied. The live weight, number and average mass of eggs in transgenic birds were higher than similar parameters in native quails. The fatty-acid composition of egg lipids in transgenic quails do not depend on such genetic modification.
Keywords: quail, egg, transgen, bovine growth hormone gene, fat acids.
Today, poultry breeding is based on selection of the best stock from high-productive families and populations, which though takes a long time. Transgenesis is one of innovative approaches for genetic improvement of poultry. Economic benefits promote its development in many countries even despite of methodological difficulties (1-3).
Integration of foreign genes into plant or animal genome is often a probabilistic process. Today, it is not always possible to integrate the genetic structure into a particular point of the genome, to predict functioning of neighboring genes, and, therefore, to expect response of the whole genome to introduction of the foreign material. Transgenesis in much the same as mutation, which is largely directed but still full of uncertainties. On the one hand, it expands the opportunities of creation animals with modified traits many of which may be interesting to a breeder, on the other – it complicates the procedure, especially when the integration of gene constructs associated with expression of bioactive substances-regulators significantly affecting many metabolic processes.
The purpose of this work was studying the effects of the integrated gene construct on economically valuable features in the offspring of genetically modified quail.
Technique. Investigations were carried out in 2001-2010 upon quail the Estonian breed bred in the vivarium of SUE “Zagorskoe Experimental and Production Farm of the All-Russia Research and Development Institute of Poultry Breeding of the RAAS”(Moscow province). (4). The bird was kept in individual cages at unlimited supply with water and feed.
The transgenic birds were obtained by microinjection of exogenous DNA into the germinal disk of eggs (5, 6). Injected gene construct pMTbGH (2½att) was provided by the Institute of Molecular Biology of the RAS (Moscow) (7).
The quails used in this study were the descendants of 20 pairs from families with high rates of egg production selected upon estimates of previous generations (8, 9). Experimental group consisted of descendants (18th-33rd generations) of transgenic parents; the control group were the Estonian quails of 15 generations not subjected to genetic modification. The offspring was obtained by natural pairing of particular individuals in both groups. Each individual from the experimental group was designated by a code in the computer database, which allowed to trace its origin starting from primary transgenic ancestors. Birds from the control group were not analyzed for pedigree.
To obtain the next generation of quail, eggs were incubated in laboratory conditions. In both groups, the hatched chicks were weighed individually at the age of 1 day, 1-, 7-, and 20 weeks (separated by gender). Along with it, the individual egg productivity and the weight of each laid egg were estimated during 10 months of productive period. A total number of all weighted eggs amounted to 135-150 thousand in each group. The bird and eggs were weighed to the nearest 0,01 g. The primary data were recorded in journals of registry and account, as well as in computer database used for further sorting and processing.
Eggs for morphological and biochemical analysis were collected during 5 adjacent days in the beginning of productive period, as well from the bird aged 3, 6 and 10 months. 80 eggs from the 30th generation of quails were analyzed in both groups. The weight of an egg was assessed by weighting a whole egg, egg shell and yolk up to 20 mg; protein content was calculated as the difference between weight of a whole egg and weight of both egg shell and yolk. The content of fatty acids in lipids of yolk was determined by gas-liquid chromatography (Fractovap-2450 by “Carbo Erba”, Italy). The analysis was performed using methyl esters – derivatives of fatty acids providing high separation efficiency at lower temperatures and shorter time of analysis (10).
Statistical processing of data was performed in Microsoft Excel with definition of Student's t-test.
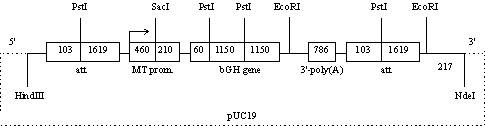
Results. The main structural component of the gene construct MTbGH (2½att) (Fig. 1) is a natural bovine growth hormone gene (bGH gene) containing introns and controlled by the mouse metal-thionine promoter (MT prom.). The promoter is activated in bird organism by zinc ions and other heavy metals. The activated promoter causes expression of bovine somatotropin gene. In transgenic quails carrying this construct, the synthesis of this hormone was demonstrated previously (5, 11).
|
Fig. 1. Physical map of the plasmid pMTbGH (2xatt) (shown sites of restriction with endonucleases) used as a gene construct to obtain genetically modified quail (description - see “Results”). |
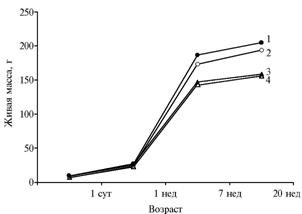
Transgenic quails exceeded the control bird by live weight over the 15 studied generations (Fig. 2), and these differences were more significant in females. At the age of 1 day and 1 week, experimental bird exceeded control by, respectively, 21 and 13%. Average weight of 1-day-old quails of the experimental group was 9,1 g, in control – 7,4 g. At the age of 7 and 20 weeks, significant differences were observed only in females – respectively, 8 and 6% (p <0,001).
|
Fig. 2. Live weight of quails the Estonian breed: intact (2, 4) and transgenic birds carrying bovine somatotropin gene (1, 3): 1, 2 — females; 3, 4 — males (in average for 15 generations) (SUE “Zagorskoe Experimental and Production Farm of the All-Russia Research and Development Institute of Poultry Breeding of the RAAS”(Moscow province, 2001-2010). Note: |
Weight of the 1-day-old chicks is greatly dependant on weight of hatching eggs. In this experiment, increased weight of eggs contributed to higher weight of transgenic 1-day-old quails (r = +0,810). All these chicks were mobile and active, showed response to sounds, had a normal soft stomach and clean cloacae. Feathers were soft, smooth, shiny, properly pigmented (brown with two pale stripes on the back), strong legs and beak, bright eyes. The appearance of adults was similar with control.
Live weight of transgenic quails varied from generation to generation, but the trend line for this indicator at the age of 20 weeks showed no downward trend (minor slope, R2 = 0,019) (Fig. 3). Changes in average live weight in some broods were possibly associated with keeping conditions but not with characteristics of their genotype.
|
Fig. 3. Live weight of quail females transgenic on bovine somatotropin gene, in different generations depending on age: a – 1 day, b – 1 week, c – 7 weeks, d – 20 weeks (SUE “Zagorskoe Experimental and Production Farm of the All-Russia Research and Development Institute of Poultry Breeding of the RAAS”(Moscow province, 2001-2010). The top graph corresponds to the trend line of live weight at the age of 20 weeks. |
Weight of eggs laid by transgenic quails was greater than that in control (Fig. 4). This trend persisted in all studied generations. Along with it, the experimental group produced more eggs than control birds. Weight of eggs is one of key indicators of egg productivity; it is primarily determined by species of birds and size of females. A normal weight of quail egg is 10-12 g. This parameter varies in different breeds within species by 20% or more, which provides great opportunities for breeding work. In this experiment, average weight of eggs laid by the 30th generation of transgenic quail amounted to 13,0 g at the peak of productivity. The spread rate for these individuals was from 11,3 to 15,0 g (32,7%).
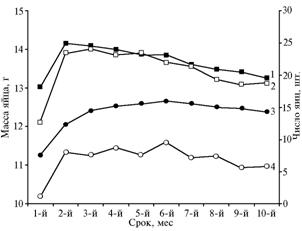 |
Fig. 4. Number (1, 2) and weight (3, 4) of eggs laid by females of quail the Estonian breed in productivity period: 1, 3 — transgenic quail, 2, 4 — intact quail (in average for 15 generations) (SUE “Zagorskoe Experimental and Production Farm of the All-Russia Research and Development Institute of Poultry Breeding of the RAAS”(Moscow province, 2001-2010). |
During the experiment, it wasn’t always possible to provide optimal conditions and the necessary quality of food, which had a greater impact on productivity of best lying hens in the experimental group than in control.
In the experimental group, increased egg weight (by 15,0 g) was the result of the proportional increase in weight of its constituent parts - shell, albumen and yolk. The share of the yolk was 29,0-33,0%, protein - 58-61%, the shell - 9,0-10,0%. Shells of eggs in both groups usually had a characteristic motley color with brown spots.
It is known that contents of major components of avian eggs (proteins, lipids, carbohydrates, water, calcium, phosphorus, iron, sodium, potassium, chlorine, magnesium, copper, sulfur) is low dependent on feeding and other external factors. However, other biochemical parameters (contents of vitamins, fluoride, manganese, iodine, oleic, linoleic and linolenic acids) are somewhat variable.
In control quails, the proportion of saturated fatty acids in eggs amounted to 41,35, monounsaturated – 41,08, polyunsaturated – 17,59%. In the experimental group, the sum of saturated and polyunsaturated fatty acids in yolk lipids were, respectively, by 1,77 and 10,35% higher, monounsaturated fatty acids - by 6,26% lower than in control. These differences emerged mainly owing to increased contents of stearic (by 12,30 %) and linoleic (by 10,70%) acids and reduced content of oleic acid (by 6,40%) in eggs of quails from the experimental group. In this case, the mass fraction of oleic acid varied from 31,40 to 37,00%, linoleic acid - from 19,90 to 17,30%. Content of fatty acids in quail eggs didn’t depend on age of laying hens. In the experimental group, the content of stearic acid in eggs from hens aged 3 and 6 months was, respectively, by 13,61 and 20,75%, linoleic acid - by 13,21 and 9,83% higher than in control. The proportion of oleic acid in eggs from 6-months-old experimental hens was found to be 12,14% lower than in control. In both groups, changes in weight of eggs were accompanied by fluctuations in contents of individual fatty acids in yolk lipids (mainly – fatty acids with 18 carbon atoms: stearic, oleic, linoleic and linolenic acids). Modified fatty acid composition of lipid yolk is an established fact, however, it’s not clear what did they come from and, particularly, if they were the result of transgenic nature: there are many factors affecting lipid composition of birds’ eggs – nutrition, housing, health, etc. The obtained data about biochemical composition of quail eggs (contents of water, dry matter, phosphorus, vitamins A, E, B, amino acid composition of protein) (12) showed no regular differences between eggs from descendants of intact quails and eggs from transgenic birds (only minor changes of some indicators).
Thus, transgenic quails carrying bovine growth hormone gene develop higher live weight, number and average weight of eggs than intact bird. Biochemical and morphological analysis of eggs laid by transgenic quails revealed no differences from that of non-transgenic birds.
REFERENCES
1. Kwon M.S., Koo B.C., Choi B.R., Park Y.Y., Lee Y.M., Suh H.S., Park Y.S., Lee H.T., Kim J.H., Roh J.Y., Kim N.H. and Kim T., Generation of Transgenic Chickens that Produce Bioactive Human Granulocyte-Colony Stimulating Factor, Mol. Reprod. Dev., 2008, vol. 75, no. 7, pp. 1120-1126.
2. Lee S.H., Gupta M.K., Han D.W., Han S.Y., Uhm S.J., Kim T. and Lee H.T., Development of Transgenic Chickens Expressing Human Parathormone under the Control of a Ubiquitous Promoter by Using a Retrovirus Vector System, Poult. Sci., 2007, vol. 86, no. 10, pp. 2221-2227.
3. Volkova N.A., Zinov’eva N.A., Volkova L.V. and Ernst L.K., Retroviral-Mediated Gene Transfer as an Effective Tool for the In Vitro Genetic Transformation of Chicken Embryonic Cells and Production of Transgenic Chickens, Genetika, 2006, vol. 42, no. 1, pp. 84-88.
4. Kochetova Z.I., Belyakova L.S., Filonenko V.I. and Chintsova A.I., Razvedenie i soderzhanie perepelov (Breeding and Keeping Quails), Stolyar T.A., Ed., Sergiev Posad, 2006.
5. Karapetyan R.V., Phenotypical Development of Transgenic Characteristics in Quails Grown from Ovicells Injected with Allogenic DNA, S.-kh. biol., 1997, no. 4, pp. 89-96.
6. Fisinin V.I., Ernst L.K., Karapetyan R.V., Matveenko N.P., Zazykina T.V., Ziadinova O.F. and Zhdanov A.B., The Method for Improving Productivity of Poultry. The All-Russia Research and Technological Institute of Poultry Breeding, C1 6 A 01 K 67/02 RU Patent # 2061366, Moscow,. 1996.
7. Zhadanov A.B., The Development of Vectors for Expression of Foreign Genes in Transgenic Animals, Extended Abstract of Cand. Sci. Dissertation, Moscow, 1991.
8. Karapetyan R.V., Korshunova L.G., Korshunov K.R., Amdii E.M. and Ernst L.K., Phenotypic Features of Quails the Descendants of Birds Transgenic for Bovine Growth Hormone Gene, in Tez. dokl. IIMezhd. nauch. konf. “Biotekhnologiya v rastenievodstve, zhivotnovodstve i veterinarii” (Abstracts of Papers, II Int. Sci. Congress “Biotechnology in Agriculture, Animal Husbandry and Veterinary”), Moscow, 2000, pp. 140-141.
9. Korshunova L.G., Karapetyan R.V., Korshunov K.R., Serikova V.A. and Ziadionva O.F., Comparative Evaluation of Egg Productivity in Descendants of Transgenic and Gray Quails the Estonian Breed, Sb. nauch. tr. VNITIP, Sergiev Posad, 2001, vol. 76, pp. 78-85.
10. Arkhipov A.V., Izuchenie lipidov i lipidnogo obmena u ptits s primeneniem tonkosloinoi i gazozhidkostnoi khromatografii: Metod. rekom (Methodological Recommendations on Studying Lipids and Lipid Metabolism in Birds Using Thin-Layer and Gas-Liquid Chromatography), Moscow, 1973.
11. Korshunova L.G. and Karapetyan R.V., Using PCR for Identification of Growth Hormone Gene Integrated in Genome of Quail, in Mat. mezhd. simp. “Molekulyarnaya genetika i biotekhnologiya v otsenke i izmenenii genomov s.-kh. zhivotnykh” (Proc. Int. Congress “Molecular Genetics and Biotechnology in Genome Modification of Farm Animals”), St.Petersburg – Pushkin, 1994, pp. 18-19.
12. Korshunova L.G., Egg Quality of Transgenic Quail, Ptitsevodstvo, 2009, no. 4, pp. 35-36.
All-Russia Research and Technological Institute of Poultry Breeding, RAAS, Moscow province, Sergiev Posad 141311, Russia |
Received January 31, 2011 |