УДК 619:615.31:615.012.1577.0
ANTIPARASITIC ACTIVITY OF FAMECTIN AND SOME COMPOUNDS OF DIFFERENT CHEMICAL NATURE
M.Kh. Dzhafarov, M.N. Myrzaev, I.V. Zavarsin
Semisynthetic derivatives of avermectins, famectin, and some compounds of steroidal and other chemical nature were tested as biocide agents. The antiparasitic activity was determined with application of oilgohets Tubificidal tubifex as test-objects. Some of these compounds demonstrate the effective antiparasitic action.
Keywords: antiparasytic activity, semisynthetic 16-membered macrolides, adermectin, famectin, steroids, transformed proline, oligochaeta.
Avermectin medications are known to provide simultaneous insecticide, acaricide and nematicide effects at relatively low doses, which makes unnecessary multiple treating animals during mixed infestations. However, some researchers observed the development of resistant forms of parasites and reducing the efficiency of many well-known antiparasitic drugs of this group (1, 2), so it becomes of a great theoretical and practical interest to obtain new promising broad-spectrum antiparasitic medications. The authors expected to find such properties in macrolides (1, 2), steroids (3) and cis-5-fenilprolin (4).
It’s fairly often that the combined use of well-known antiparasitic and antibacterial compounds (benzimidazole derivatives, macrolides, penicillins, glucosamine, nicotinoids) (5) doesn’t change the fundamental molecular mechanisms of their individual action, but this allows a relatively quick response to development of resistance and prevent it for some (usually short) period. Steroids are in focus, because these endogenous lipophilic metabolites can be used as a platform - the molecular carrier of bioactive antibacterial or antiparasitic structural fragments covalently bound to the steroid molecule and purposefully brought to organs and target cells. Such carrier can transfer the active ingredient equally at the intercellular level and within the cell from its plasma membrane to the DNA. A sitosterol glycoside b-sitosterol-3-O-glucopyranoside is assumed to be one of the most promising inhibitors of the recently discovered bacterial sortase (6). Steroid compounds are also of interest owing to detection in some pathogens (germs of tuberculosis, plague, typhus, anthrax, cholera, etc.) of the non-mevalonate pathway of isopentenyl pyrophosphate biosynthesis – the important intermediate of isoprenoid synthesis (7). Interestingly that a famous fusidic acid is a 19-retrostructure isoprenoid providing bacteriostatic effect on Staphylococcus, meningococci and gonococci (8).
To solve this problem, the authors developed a series of compounds of different chemical nature (production technique of these compounds and pharmaceuticals will be protected by patents). Earlier, the authors have found the effective antiparasitic action in some semi-synthetic derivatives of 16-membered macrolides, and they have first reported about such activity found in several steroid compounds (9, 10).
The purpose of this work was studying antiparasitic effects of famectin and organic compounds of steroid and other chemical nature.
Technique. The authors compared effects of known (abamectin, closantel) and the proposed organic compounds with working names Ad (adermectin) (10), Fab (famectin), R-407, R-408, R-412, R-413, Ch-243, KUD-735 (4), as well as combined preparations containing Ad and pro-S-1.1 (10) at different ratios (1:10, 1:100). The test objects for rapid assessment of biocidal activity were oligochaetes Tubificidal tubifex (11). Acute toxicity of Fab was determined as described (12), on standard laboratory white mice with body weight 19-20 g when administered intraperitoneally (animal tests were performed according to regulations of the European Convention for the Protection of Vertebrate Animals used for experimental and other scientific purposes) (86/609 EEC).
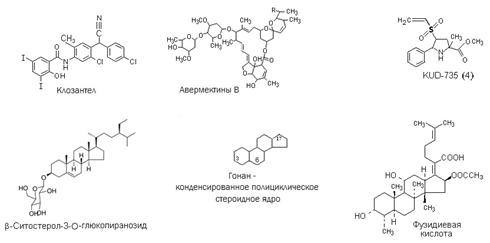
Results. Ad and Fab have a macrolide nature (result of chemical modification of 16-membered macrolides - avermectins produced by the soil bacterium Streptomyces avermitilis) (13). Compounds with the symbols R-407, R-408 and so on, Ch-243 and pro-S-1.1 are the substances of steroid nature (3, 9) containing the active functional groups in positions C-3 and C- 20 or C-6, KUD-735 - transformed proline with the potential center inhibiting sortase SrtA isoform of Staphylococsus aureus (4) (Fig.).
|
Chemical structure of come compounds with antiparasitic activity. R — methyl or ethyl group. |
Antiparasitic activity of selected compounds was studied: 5,0 ug/ml abamectin paralyzes 50% oligochaeta after 30 minutes of action. A similar effect was observed in closantel (10 ug/ml, 180 min), Fab and Ad (5,0 ug/ml, 30 minutes), as well as at the combined use of Ad and pro-S-1.1 (1:10 and 1:100) - at concentrations of 10 and 5 ug/ml, respectively (Table).
Acute toxicity was assessed by LD50 and LD100 in white mice: for Ad, it equaled 35,96 mg/kg and 69,44 mg/kg, respectively, for Fab – 36,45 and 72,31 mg/kg. These doses are comparable with LD50 of commonly used avermectin-containing medications: for abamectin - 19, ivermectin - 53 and aversectin C - 90 mg/kg (14).
Comparative data on biocidal action of studied compounds of different chemical nature (macrolides, steroids and transformed proline) in the rapid test on oligochaeta Tubificidal tubifex |
||||
Studied compound |
Concentration, ug/ml |
Time of action, minutes |
||
30 |
60 |
180 |
||
Abamectin (Ab) |
5,0 |
++ |
+++ |
+++ |
Adermectin (Ad) |
5,0 |
++ |
++ |
+++ |
Closantel |
5,0 |
0 |
+ |
+ |
Fab (famectin) |
5,0 |
++ |
++ |
+++ |
R-407 |
5,0 |
0 |
0 |
0 |
R-408 |
5,0 |
0 |
0 |
0 |
R-412 |
5,0 |
0 |
0 |
0 |
R-413 |
5,0 |
0 |
0 |
0 |
Ch-243 |
5,0 |
0 |
+ |
+ |
KUD-735 |
5,0 |
0 |
+ |
+ |
Combination Ad + pro-S-1.1: |
|
|
|
|
1:10 |
5,0 |
+ |
++ |
+++ |
1:100 |
5,0 |
++ |
++ |
+++ |
Note. Description of compounds – see “Technique”. 0 — zero effect; «+», «++», «+++» and «++++» — paralysis of, respectively, less than 50 %, 50-60 %, 60-80 % and 80-100 % individuals. |
||||
Intoxication was clinically manifested in all mice as muscle tremors and convulsions, dystaxia followed by strong suppression: the animals were lying with no reaction to external irritants and died after 20-30 minutes. Autopsy revealed no visible pathological changes in the dead animals and ones euthanized at the end of the experiment.
Thus, the tested substances provide the effect on organisms corresponding to the II class of hazard according to standards of GOST 12.1.007-76 (highly-hazardous matters).
The studied compounds contain the structural fragment with a keto-group or a polar functional group (-OH, -SH, =NH, =O, =S, etc.). Dreiding models of these compounds show such functional groups located at an angle of 90° relative to the central plane of a cyclic structure of macrolide or steroid core, which configuration apparently provides the interaction with target receptors.
The experimental forms of commercial antiparasitic products based on Ad and Fab were developed and found to be effective in preliminary tests. The Ad-based medication was found to be highly stable: it maintained antiparasitic activity after the 1-year storage. The data on pharmacodynamics and pharmacokinetics of the developed Fab- and Ad-based formulations will be published in a separate report.
Thus, there were studied substances of different chemical nature (macrolides, steroids, transformed proline and macrolide-steroid composition) and the significant antiparasitic effect was established in adermectin (Ad), famectin (Fab), Ch-243, as well as in compositions Ad+pro-S-1.1 (with different ratios of components). These formulations can be suggested as promising active ingredients of biocidal medications for animal husbandry.
REFERENCES
1. Strachunskii L.S. and Kozlov S.N., Makrolidy v sovremennoi klinicheskoi praktike (Macrolides in Modern Clinical Practice), Smolensk, 1998.
2. Macrolide Antibiotics. Chemistry, Biology and Practice, Omura S, Ed., New York: Elsevier Science, 2002.
3. Dzhafarov M.Kh., Zaitsev S.Yu. and Maximov V.I., Steroidy. Stroenie, poluchenie, svoistva i biologicheskoe znachenie. Primenenie v meditsine i veterinarii (Steroids. Structure, Synthesis, Properties and Biological Role. Application in Medicine and Veterinary), St.Petersburg, 2010.
4. Kudryavtsev K.K., Bentley M.L. and McCafferty D.G., Probing of Cis-5-Phenyl Proline Scaffold as a Platform for the Synthesis of Mechanism-Based Inhibitors of the Staphylococus aureus Sortase SrtA Isoform, Bioorg. Med. Chem., 2009, vol. 17, pp. 2886-2893.
5. Lopatina Yu.V. and Eremina O.Yu., Neonicotinoid Insecticides in Veterinary Practice, S.-kh. biol., 2005, no. 6, pp. 14-24.
6. Suree N., Yi S.W., Thieu W., Marohn M., Damoiseaux R., Chan A., Jung M.E. and Clubb R.T., Discovery and Structure-Activity Relationship Analysis of Staphylococcus aureus Sortase A Inhibitors, Bioorg. Med. Chem., 2009, vol. 17, pp. 7174-7185.
7. Rohdich F., Bacher A. and Eisenreich W., Isoprenoid Biosynthetic Pathways as Anti-Infective Drug Targets, Biochem. Soc. Trans., 2005, vol. 33, pp. 785-791.
8. Maskovskii M.D., Lekarstvennye sredstva (Medications), Moscow, 2010.
9. Dzhafarov M.Kh., Zaitsev S.Yu., Mirzaev M.N. et al., Synthesis and Biological Activity of Steroid Dihydropyrazoles, Rossiiskii immunologicheskii zhurnal, 2008, vol. 2, no. 11/2-3, p. 192.
10. Dzhafarov M.Kh., Zaitsev S.Yu., Mirzaev M.N., Urazaev D.N. and Maximov V.I., Antiparasitic Activity of Adermectin and Compounds of a Steroid Nature, Russ. Agr. Sciences, 2010, vol. 36, no. 2, pp. 130-132.
11. Drinyaev V.A., Chizhov V.N., Kovalev V.N. and Mirzaev M.N., A Method for Determination Nematicide Activity of Avermectins, Patent RF ¹ RU 2013053 C1; A01N63/00, published 1994.
12. Khabriev R.U., Denisov I.N., Gerasimov V.B., Kukes V.G. et al., Rukovodstvo po eksperimental’nomu (doklinicheskomu) izucheniyu novykh farmakologicheskikh veschestv (A Manual on Experimental Pre-Clinical Investigation of New Pharmaceuticals), Moscow, 2005.
13. Ikeda H. and Omura S.,l Avermectin Biosynthesis, Chem. Rev., 1997, vol. 97, no. 7, pp. 2591-2610.
14. Gul’chinskaya T.S., Avermektinsoderzhaschie in’ektsionnye lekarstvennye sredstva na rossiiskom rynke vetpreparatov (Avermectin Injection Drugs on the Russian Market of Veterinary Medications) http://www.vettorg.ru/magazines/?magid=2&year= 2001&issid=9&artid=91.
K.I. Skryabin Moscow State Academy of Veterinary Medicine and Biotechnology, Moscow 109472, Russia, |
Received January 31, 2011
|