УДК 591.05:577.161.3
ABOUT α-TOCOPHERYLQUINONE PARTICIPATION IN NAD+-DEPENDENT REGULATION OF FINAL STAGE OF GLUCOSE CLEAVAGE
V.I. Dudin
In the experiments with incubation of dry fast-acting baker's yeast Saccharomyces cerevisiae S15С (OXY) in incomplete medium with D-glucose the author established, that the addition to medium of NAD+ increases the speed of pyruvate formation. The combination of NAD+ and α-toco-pherylquinone changes markedly in medium the dynamics of acetaldehyde content. The use of lithium lactate as substrate permits to detect two maximum of pyruvate concentration as result of action of two lactate dehydrogenases, one of which (14 h) is inhibited by NAD+ and α-tocopherylquinone together, the other (36 h) — by these agents separately.
Keywords: dried baker`s yeast Saccharomyces cerevisiae, glucose, acetic aldehyde, α-tocopherolquinone, pyruvic acid and acetic aldehyde formation, fermentation, lactate dehydrogenases, control of activity.
The baker's yeast Saccharomyces cerevisiae (fresh and dry long-stored) during the experiment with incubation in the incomplete culture medium with D-glucose has shown that D,L-α-tocopherylquinone (the product of free radical oxidation of vitamin E) accelerates the formation of pyruvate from glucose (1). NAD+-dependent dehydrogenases (such as lactate dehydrogenase, alcohol dehydrogenase, D-glyceroal-dehydro-3-phosphate dehydrogenase) are composed of similar protein fragments - NAD+-binding domains (2). Their binding is controlled by NAD+, and high sensitivity of these enzymes to regulatory factors is possibly based on a-tocopherylquinone participation in this process.
Lactate dehydrogenases of mammalians are known to be specific to L(+)-lactate (meat-and-milk acid). The yeast lactate dehydrogenase (L-lactate: ferricytochrome c oxidoreductase, EC 1.1.2.3) is identical to cytochrome b2 and it shows a substrate stereospecificity. The enzyme is a bifunctional tetramer; each subunit consists of two main domains - the domain of cytochrome b and the domain containing flavin mononucleotide (3). The enzyme utilizing L-lactate was found to be significantly distinct from the enzyme catalyzing oxidation of D-lactate (D-lactate: ferricytochrome c oxidoreductase, EC 1.1.2.4) (4).
The purpose of this work was studying the effect of α-tocopherylquinone and NAD+ on pyruvate and acetaldehyde contents in the medium during the fermentation of glucose or lithium lactate by yeast Saccharomyces cerevisiae.
Technique. The study was performed with the use of commercial dry baker's yeast Saccharomyces cerevisiae the strain S15S (OXY) (“Lesaffre”, France). D,L-α-tocopherylquinone was obtained by oxidation of D,L-α-tocopherylquinone with nitric acid (5) and purified on a silica gel column under the control of high-performance liquid chromatography (HPLC) (6).
In the first variant, 4 g D-glucose was dissolved in 200 cm3 water. The resulting solution was poured in two bulbs of 200 cm3 volume (100 cm3 in each bulb). The test sample was added with NAD+ (“Sigma-Aldrich”, Germany) and a-tocopherylquinone (0,2 umol/cm3) solubilized in 10 ml water using Tween 20 (150 mg) (“Merck”, Germany). The control sample was added with the same quantity of Tween 20 and water, as well as NAD+. The following doses of NAD+ (final concentrations) were tested: 0; 0,34; 0,68; 1,37; 2,74 and 5,48 umol/cm3 of a medium. 0,75 g yeast was introduced in each flask. The mixtures were incubated in a water bath with shaker at 30 °C. Initial samples were taken in 12 minutes after the start, then the flasks were placed back and samples (5 ml) were collected in 3,5; 7; 14; 24; 36; 48; 60; 72 and 84 h. After precipitating a protein with 1,25 ml 10% HPO3 the samples were centrifuged in the refrigeration centrifuge K-24 (“Janetzki”, Germany) at 10 000 g and 4 °C. The second and third variants: the substrate – lithium salt of D,L-lactate (“Sigma”, USA) at the content of 90 umol/cm3. The second variant: the control sample was added with only 150 mg Tween 20, the test sample was added with Tween 20 (150 mg) and 0,0227 mmol D,L-a-tocopherylquinone (20 umol/cm3). The third variant: both samples were also added with 0,6 mmol NAD+ (5,48 umol/cm3). 0,85 g yeast was introduced in each flask.
The content of pyruvic acid was determined by its ability to form 2,4-dinitrophenyl hydrazones, which were purified by the reverse extraction from toluene solution into an aqueous solution of soda (Na2CO3) and then transferred to aci-form by adding sodium hydroxide solution (7). Acetaldehyde was isolated as 2,4-dinitrophenyl hydrazone from the toluene solution remaining after isolation of the acidic 2,4-dinitrophenyl hydrazones of pyruvic acid. Toluene was evaporated and 2,4-dinitrophenyl hydrazones of acetaldehyde were isolated by double chromatography with a thin bound layer of silica gel L (“Lachema”, Czech Republic) and benzene as a carrier. Hydrazones were extracted from silica gel using methanol. The content of aldehyde was measured on the spectral colorimeter Specol-11 (“Carl Zeiss”, Germany) at l = 340 nm.
The second and third variants: the content of hydrazones of pyruvate in samples was determined after purification by thin-layer chromatography. The synthesis of 2,4-dinitrophenyl hydrazones was followed by extraction of them with toluene and the reverse extraction into an aqueous solution of soda (7). 2,4-dinitrophenyl hydrazones were extracted with toluene after neutralization with 2N HCl to low acidity (pH 5.0). The solvent was evaporated, hydrazone of pyruvate was isolated from the thin layer of silica gel using the mixture of 10% ethanol in benzene (Rf = 0,50) as a carrier.
The data were statistically processed upon common principles of variation statistics (8) in Microsoft Excel.
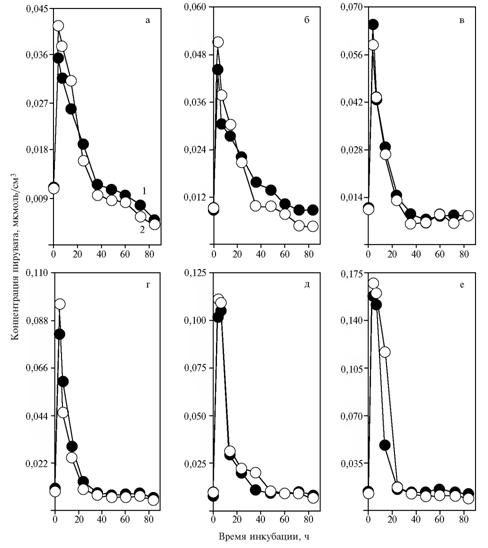
Results. Increasing the dose of NAD+ in the medium with glucose contributed to rise in pyruvate content depending on time of incubation (Fig. 1). NAD+ doses of 2,74 and 5,48 umol/cm3 (Fig. 1, e, f) caused a shift of the peak from 3,5 hours to the period between 3,5 and 7 h. At the same time, adding a-tocopherylquinone provided no significant changes nor in pyruvate content in medium neither in timing of its peaks relative to the control. Inhibition of pyruvate synthesis by a-tocopherylquinone was established only after adding NAD+ (5,48 umol/cm3) between the 7th and 14th hours of incubation (Fig. 1, f).
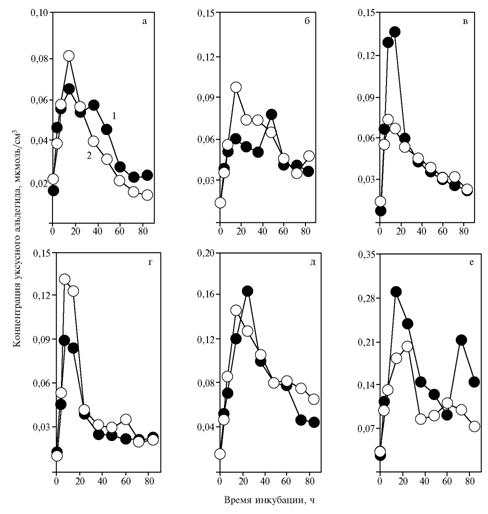
In the case of a relatively mild increase in NAD+ content in medium, a-tocopherylquinone significantly affected the dynamics of acetaldehyde content. This is clearly seen in multidirectional action of a-tocopherylquinone on acetaldehyde content when adding NAD+ at doses of 0,68 (Fig. 2, c) and 1,37 umol/cm3 (Fig. 2, d). These findings can be regarded as the ability of a-tocopherylquinone to regulate acetaldehyde metabolizing depending on NAD+ content in the medium.
|
| Fig. 1. The concentration of pyruvate during fermentation of glucose by baker’s yeast Saccharomyces cerevisiae the strain S15C (OXY) after introducing NAD+ to a medium: а, б, в, г, д, е — respectively, 0; 0,34; 0,68; 1,37; 2,74 and 5,48 umol/cm3; 1 and 2 — without introducing α-tocopherylquinone to a medium.
Note: |
Earlier, NAD+-binding domain has been studied in alcohol dehydrogenase of the horse liver (9). This enzyme was found to be the Zn2+-dependent homodimer; each its protomer contains a coenzyme-binding domain and catalytic domain. It has been studied the mechanism of domain closure induced by NAD+. Simulation and precise X-ray analysis have confirmed that the loop of the domain interface prevents its closure at absence of NAD+. The subunits cooperate to support the role of the loop as a blocker of domain closure at absence of NAD+ during the period when other subunits are closed by NAD+. As a result, the enzyme can be extremely sensitive to preventing or controlling factors. In particular, functioning of the catalytic domain is influenced by bismuth preparation (10) capable to inhibit the activity of alcohol dehydrogenase. It can substitute only a half of Zn2+ (approximately one Zn2+ per monomer). Sulfur (5,5 ppm) inhibits alcohol dehydrogenase and catalase too, while ditiotreitol enhances the action of the element (11).
|
| Fig. 2. The concentration of acetaldehyde during fermentation of glucose by baker’s yeast Saccharomyces cerevisiae the strain S15C (OXY) after introducing NAD+ to a medium: а, б, в, г, д, е — respectively, 0; 0,34; 0,68; 1,37; 2,74 and 5,48 umol/cm3; 1 and 2 — without introducing a-tocopherylquinone to a medium.
Note: |
|
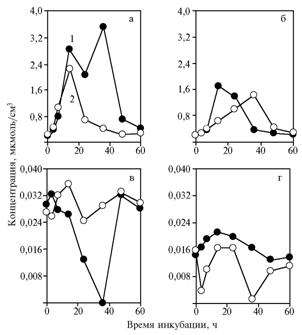
| Fig. 3. The concentration of pyruvate (а, б ) and acetaldehyde (в, г) during fermentation of lithium lactate by baker’s yeast Saccharomyces cerevisiae the strain S15C (OXY) without (а, в) and with introducing NAD+(б, г) to a medium: 1 and 2 — respectively, without and with introducing α-tocopherylquinone to a medium.
Note: |
Lactate is a product of one metabolic pathway (glycolysis) and the substrate for another one (respiration). It plays the role of extracellular and intracellular shuttle involved in transfer of oxygenated and gluconeogenetic substrates (12). For example, the exchange of lactate between white (glycogenic) and red (oxidative) muscle fibers, between working skeletal muscle and heart tissue, between the release of lactate and gluconeogenesis. Mitochondrial lactate-pyruvate exchange is driven by lactate dehydrogenase providing oxidation of lactate in actively respiring cells. Glycolysis and oxidation of lactate in cells cause fast flow of substrates and the maintenance of redox balance between cytosol and mitochondria. Lactate dehydrogenases converting lactate to pyruvate were found in most organisms; this group of proteins is quite diverse in amino acid sequence, catalytic properties and substrate specificity (13).
In this experiment, using lithium lactate as a substrate resulted in emergence of two peaks on the chart of pyruvate concentration (14 and 36 h). Adding NAD+ to a medium (Fig. 3, b, d) reduced the intensity of fermentation, probably due to inhibitory properties of this coenzyme for lactate dehydrogenase.
Thus, the yeast S. cerevisiae the strain S15S (OXY) has two lactate dehydrogenases with different properties. One of them is inhibited by both a-tocopherylquinone and NAD+ simultaneously, the other - by the same agents separately. The first is manifested quickly (after 14 h), the second - slowly (after 36 h). Most likely these are L- and D-lactate dehydrogenases. The coincidence of pyruvate concentration peak with the minimum content of acetaldehyde is of particular interest. Such relationship of substrates can be provided by growing activity of acetoin synthesis by pyruvate decarboxylase. It is known that the addition of pyruvate to fermenting yeast leads to formation of acetoin (14). In this experiment, the similar emergence of pyruvate in the reaction mixture was the result of action of yeast lactate dehydrogenases.
Yeast lactate dehydrogenase and alcohol dehydrogenase can be inactivated by attacking with free radicals, which is less intense in the oxygen-free medium (15). Ascorbate can protect both enzymes from such attacks. This fact underlies the common opinion about the influence of antioxidants on enzyme activity. Tocopherylquinone is a strong renewable antioxidant capable to restoration in the respiratory chain (16). At the same time, the findings presented in this report can’t be explained by antioxidant effects of a-tocopherylquinone provided by working mitochondria.
It is shown that quinones can act as oxidants of a flavoprotein (17). In the rumen anaerobic bacterium Butyrivibrio fibrisolvens and in Escherichia coli α-tocopherylquinone is reduced to the corresponding quinol during the reaction catalyzed by a flavoprotein enzyme at presence of reducing equivalents NADH (18). Findings of this study make it necessary to reveal the mechanism of α-tocopherylquinone action which involves the participation of NAD+.
So, during the fermentation of D-glucose by baker's yeast Saccharomyces cerevisiae S15S (OXY), the rate of pyruvate formation was growing in case of adding NAD+ to a medium. The combined addition of NAD+ and α-tocopherylquinone significantly changed the dynamics of acetaldehyde content. Using lithium lactate as a substrate resulted in emergence of two peaks of pyruvate content reflecting the action of two lactate dehydrogenase.
REFERENCES
1. Dudin V.I., About Participation of a-Tocopherylquinone in Glucose Fermentation by Saccharomyces cerevisiae, S.-kh. biol., 2010, no. 2, pp. 34-39.2. Anisimov A.A., Osnovy biokhimii (Fundamentals of Biochemistry), Moscow, 1986.
3. Zvyagil’skaya R.A. and Kotel’nikova A.V., Structure and Functional Activity of Yeast Mitochondria, in Itogi nauki i tekhniki VINITI. Seriya “Biologicheskaya khimiya” (Scientific and Technical Summary of the All-Russia Institute of Scientific and Technical Information. Series “Biological Chemistry”), Moscow, 1991, vol. 36.
4. Wilkinson J., Izofermenty (Isoenzymes), Moscow, 1968.
5. Rao G.H.R., Keich T.P. and White J.G., Preparation, Separation and Characterization of Vitamin E Quinone, J. Chromat., 1980, vol. 196, no. 3, pp. 506-511.
6. Dudin V.I., Biokhimiya vitamina E i svyazannykh s nim biologicheski aktivnykh veschestv (Biochemistry of Vitamin E and Related Bioactive Substances), Moscow, 2004.
7. Petrun’kina A.M., Prakticheskaya biokhimiya (Practical Biochemistry), Leningrad, 1961, pp. 384-390.
8. Merkur’eva E.K., Biometriya v zhivotnovodstve (Biometry in Animal Husbandry), Moscow, 1964.
9. Hayward S . and Kitao A., Molecular Dynamics Simulations of NAD+-Induced Domain Closure in Horse Liver Alcohol Dehydrogenase, Biophys. J., 2006, vol. 1, no. 91/5: pp. 1823-1831.
10. Jin L., Szeto K.Y., Zhang L., Du W. and Sun H., Inhibition of Alcohol Dehydrogenase by Bismuth, Environ. Toxicol., 2004, vol. 19, no. 4, pp. 372-386.
11. Getkauskaite A., Pessala P. and Södergren A., Elemental Sulfur Toxicity In Vivo and In Vitro to Bacterial Luciferase, In Vitro Yeast Alcohol Dehydrogenase, and Bovine Liver Catalase, Mol. Cell. Biol., 2004, vol. 24, no. 9, pp. 3874-3884.
12. Brooks G.A., Lactate Shuttles in Nature, Microbiologia, 1996, vol. 12, no. 3, pp. 411-416.
13. Cristescu M.E. and Egbosimba E.E., Evolutionary History of D-Lactatede-Hydrogenases: a Phylogenomic Perspective on Functional Diversity in the FAD Binding Oxidoreductase/Transferase Type 4 Family, J. Mol. Evol., 2009, vol. 69, no. 4, pp. 276-287.
14. Samner J.B. and Somers G.F., Khimiya fermentof i metody ikh issledovaniya (Methods and Chemistry of Enzymes), Moscow, 1948.
15. Kittridge K.J. and Willson R.L., Uric Acid Substantially Enhances the Free Radical-Induced Inactivation of Alcoholdehydrogenase, Biochem. J., 1984, vol. 95, no. 1, pp. 109-115.
16. Dudin V.I., Grischuk S.V. and Sakovtseva T.V., About Participation of a-Tocopherylquinone and Ubiquinone in Regulation of Growing Processes in Pigs and Poultry, S.-kh. biol., no. 6, pp. 53-58.
17. Dolin M., Metabolizm bakterii (Metabolism of Bacteria), Moscow, 1963, pp. 316-359.
18. Hughes P.E. and Tove S.B., Identification of an Endogenous Electron Donor for Biohydrogenation as a-Tocopherolquinol, J. Biol. Chem., 1980, vol. 255, no. 10, pp. 4447-4452.
All-Russia Research and Development Institute of Physiology, Biochemistry and Feeding, RAAS, Kaluga province, Borovsk249013, Russia |
Received February 26, 2010
|