УДК 636/639:614.31
METHODS OF SANITARY SURVEILLANCE OF LIVESTOCK PRODUCTION. III. ENZYMOIMMUNOASSAY OF GENTAMYCIN
M.A. Burkin1, G.P. Kononenko2, A.A. Burkin2
During optimization of the conditions of indirect competitive solid-phase enzymoimmunoassay the authors revealed that two species of polyclonal rabbit antibodies, obtained to previously synthesized conjugate antigens with different structure on basis of gentamycin, have equal high specificity, and sensitivity of determination is 1-10 ng/ml of gentamycin. The possibility of the use such test-system for the control of gentamycin contamination in milk and meat production is discussed.
Keywords: gentamicin, milk, meat, immunoassay.
Gentamicin (GM) is aminoglycoside antibiotic widely used in domestic and international animal husbandry as a component of medications for injection or watering to animals (1, 2). Currently, foreign researchers suggested several variants of immunoassay (3-5) to control GM contamination of milk and meat.
The purpose of this research was to obtain specific immunoreagents based on gentamicin and optimize its determination in livestock products by indirect competitive enzyme-linked immunosorbent assay (ELISA).
Technique. During the study, there were used gentamicin sulfate (G 632), a1-acid glycoprotein (GP) from bovine plasma (G 3643) (“Sigma”, USA), glutaraldehyde (“Reanal”, Hungary), organic solvents (“Fluka” , Germany), bovine serum albumin (BSA), rabbit serum albumin (RSA), gelatin (Gel), succinic anhydride, sodium periodate, sodium borohydride and antispecific enzyme conjugate prepared as described (6) of horseradish peroxidase (EC 1.11.1.7) and donkey antiserum to rabbit immunoglobulin (Russia). ELISA was performed on highly-binding polystyrene plates (“Costar”, USA) using a photometer AKI-Ts-01 (Russia). UV spectra were recorded on the device Hitachi-557 (“Hitachi”, Japan).
GM conjugates with native BSA and Gel were synthesized using glutaraldehyde and the proteins pre-succinylated by carbodiimide condensation method (sBSA, sRSA and sGel). Conjugation with GP was performed in conditions of periodate oxidation (7).
To obtain BSA-GM (100), Gel-GM (5), Gel-GM (10) and Gel-GM (25), BSA solution (5 mg in 2,0 ml water) was added with GM (6 mg) and three portions of Gel solution (8 mg = 0,05 umol in 1,5 ml water) were added with GM aliquots (4 mg in 0,4 ml water) required for corresponding molar excesses. Then, the reaction mixtures were added with 30 ul freshly prepared 2,5% glutaraldehyde, mixed on a magnetic stirrer for 2 h at room temperature, then each sample was added with 100 ml solution of sodium borohydride (2 mg/ml). The stirring was continued for 1 hour at 4 oC and then dialyzed against three changes of 1000-fold volume of 0,5% sodium chloride solution. To obtain sBSA-GM (50), sRSA-GM (50) and sGel-GM (50), solutions of BSA, RSA and Gel (respectively, 7,0; 6,5 and 8,0 mg or 0,1; 0,1 and 0,05 umol) in water (0,5 ml) were added with one drop of 1 N NaOH and 5 mg succinic anhydride (20 umol) in dimethylformamide (0,5 ml). The mixtures were placed on a magnetic stirrer for 2 h at room temperature, maintaining pH 9,0-9,5, and then dialyzed against the 1000-fold volume of 0,15 M phosphate buffer (pH 5,5). The solutions of succinylated proteins were added with water-soluble carbodiimide (9,5 mg, or ~50 umol), and after 30 min - 50-fold molar excess of GM (respectively, 2,50; 2,50 and 1,25 mg), stirred for 14 hours at room temperature and dialyzed against three changes of 1000-fold volume of 0,5% sodium chloride solution. To synthesize GP-GM (50), GP solution (4 mg or 0,1 umol in 1 ml water) was added with NaIO4 (4 mg), followed by stirring on a magnetic stirrer (30 min) and dialysis against 0,001 M acetic acid (16 h). At the end of dialysis, the oxidized protein solution was added with GM solution (2,5 mg in 0,5 ml 0.05 M carbonate buffer, pH 9,5), placed on a stirrer for 2 h; after that, 200 ml NaBN4 (2 mg/ml) was introduced into the mixture, which then were kept at periodic stirring for 2 h at 4 oC and dialyzed against three changes of 1000-fold volume of 0,5% sodium chloride solution. After the dialysis, the resulting products of all reactions were added with an equal volume of glycerol and stored at -15 ... -10 °C.
Rabbits (gray colored males with live weight 2-3 kg) were immunized with conjugates sBSA-GM (50) and BSA-GM (100). The first injection containing 100 micrograms immunogen in complete Freund's adjuvant was administered subcutaneously in 10-15 points on the back of the animals. The second and all subsequent injections containing 100 ug immunogen in saline were given at 1-month intervals. After 7 days post the second and all subsequent injections, blood samples were collected from the edge ear vein; the serum was separated, added with an equal volume of glycerol and then stored at -15 ... -10 °C.
Testing of sera, evaluation of antibody specificity, analyzing conjugates as solid-phase antigens and performance of ELISA did not differ from those described previously (8). Meat and milk samples used for the analysis were purchased from the market of Moscow.
Results. Conjugation of GM, which molecule has primary amino groups, was carried out by alternative ways: through the amino groups of proteins - in the reaction with glutaraldehyde, through a carboxyl group - in carbodiimide condensation, as well as direct binding to the aldehyde groups of pre-oxidized glycoprotein (GP). Earlier, the authors experienced the successful use of such methods for the synthesis of conjugates of other physiologically active substances (9, 10).
The facts of binding GM with proteins could be confirmed only immunochemically, because the absorption maxima of GM (11) found at l = 250 nm are overlapped in the conjugates by the broad absorption peak of the protein carrier centered at l= 280 nm. It was also impossible to clarify the concentrations of GM analytical solutions spectrometrically, since the absorption effect wasn’t detected in water- and water-acetonitrile solutions with concentrations up to 200 ug/ml, possibly owing to the extremely weak intensity.
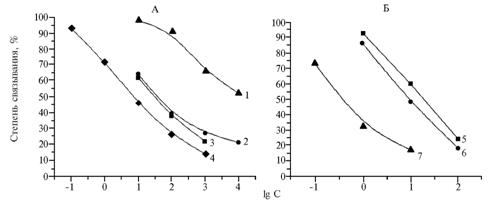
The first conjugate sBSA-GM used for immunization (50) already after the third injection to the animals (at the 2nd blood sampling) provided the formation of antibodies with working titers 1:17 000 and possibility of detecting GM in solutions up to the concentration of 10 ng/ml. Further immunization till the 4th sample of blood serum was accompanied by maintenance of the high antibody titer and enhancement of test sensitivity in an order (Fig. 1, A).
|
Fig. 1. The degree of binding antibodies to sBSA-GM(50) obtained at the 1st-4th blood sampling (1-4) with immobilized antigen sGel-GM(50) (A) and antibodies to BSA-GM(100) derived at the 1st-3rd blood sampling (5-7) with immobilized antigen Gel-GM(25) (B) at presence of gentamicin determined by the competitive indirect enzyme-linked immunosorbent assay (ELISA): BSA and sBSA, Gel and sGel, GM – respectively, normal and succinylated bovine serum albumin, normal and succinylated gelatin, gentamicin. |
Linearity of the analytical signal within the range 10-90% of antibody binding for concentrations of 1-100 ng/ml was provided only by the immobilized conjugate GP-GM obtained by heterologous synthesis, the other ones were inferior to that though immunoreactive as well (Table 1).
1. The degree of binding (%) antibodies to sBSA-GM (50) during the competitive indirect enzyme-linked immunosorbent assay (ELISA) at presence of GM and the use of antiserum of the 4th blood sampling and different immobilized antigens |
||||
Immobilized antigen (С = 0,15 ug/ml) |
GM, ng/ml |
|||
1000 |
100 |
10 |
1 |
|
GP-GM(50) |
0 |
13 |
38 |
77 |
sBSA-GM(50) |
22 |
41 |
63 |
86 |
sRSA-GM(50) |
23 |
42 |
65 |
90 |
sGel-GM(50) |
14 |
26 |
46 |
73 |
Gel-GM(5) |
Wasn’t determined |
43 |
80 |
102 |
Gel-GM(10) |
Wasn’t determined |
41 |
79 |
104 |
Gel-GM(25) |
Wasn’t determined |
49 |
80 |
99 |
Note. GM, GP, Gel, sBSA, sRSA, sGel – respectively, gentamicin, glycoprotein, gelatin, succinylated bovine serum albumin, succinylated rabbit serum albumin, succinylated gelatin. |
||||
2. The degree of binding (%) antibodies to BSA-GM (100) during the competitive indirect enzyme-linked immunosorbent assay (ELISA) at presence of GM and the use of antiserum of the 1st blood sampling and different immobilized antigens |
|||
Immobilized antigen (С = 0,15 ug/ml) |
GM, ng/ml |
||
100 |
10 |
1 |
|
GP-GM(50) |
22 |
50 |
90 |
sRSA-GM(50) |
40 |
73 |
96 |
sGel-GM(50) |
41 |
74 |
98 |
Gel-GM(5) |
24 |
60 |
90 |
Gel-GM(10) |
21 |
55 |
91 |
Gel-GM(25) |
24 |
60 |
93 |
Note. See Table 1. |
|||
The start of immune response to conjugate BSA-GM(100) was more active (Fig. 1, B). Already in the serum of the 1st blood sampling there was observed a distinct inhibition of binding with both homologous and heterologous solid-phase antigens at the concentration of GM solution of 10 ng/ml (Table 2).
The 2nd blood sampling showed a somewhat increase in test sensitivity, while the antiserum of the 3rd blood sampling allowed to reduce the concentration of antigens applied as a solid phase in ELISA along with enhancing test sensitivity by almost an order of magnitude (Fig. 1 B). With all the homologous solid-phase antigens - Cel-GM(5), Gel-GM(10), Gel-GM (25) used at a concentration of 0,05 ug/ml there was detected the clear inhibition of antibody binding achieved already for GM solution with GM content of 0,1 ng/ml (Table 3), and the relationship between the percentage of antibody binding and GM content in the solution was close to linear.
| 3. The degree of binding (%) antibodies to BSA-GM (100) during the competitive indirect enzyme-linked immunosorbent assay (ELISA) at presence of GM and the use of antiserum of the 3rd blood sampling and different immobilized antigens | |||
Immobilized antigen (С = 0,05 ug/ml) |
GM, ng/ml |
||
10 |
1 |
0,1 |
|
GP-GM(50) |
29 |
48 |
69 |
Gel-GM(5) |
16 |
34 |
76 |
Gel-GM(10) |
17 |
31 |
71 |
Gel-GM(25) |
17 |
32 |
73 |
Note. See Table 1. |
|||
|
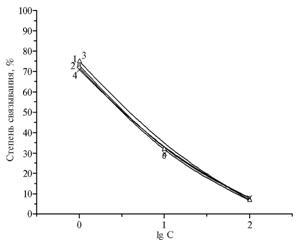
Fig. 3. Calibration graphs for the competitive indirect enzyme-linked immunosorbent assay (ELISA) of gentamicin with the use of antiserum to BSA-GM(100) of the 3rd blood sampling and immobilized antigen Gel-GM(25) obtained for PBST (1), water-acetonitrile mixture at a 10-fold dilution with PBST (2), water-acetonitrile extract of chicken muscle at a 10-fold dilution with PBST (3) and milk at a 3-fold dilution with PBST (4): BSA, Gel, GM, PBST – respectively, bovine serum albumin, gelatin, gentamicin, phosphate-buffered saline containing Tween 20. |
Antibody recognition of other aminoglycosides, including kanamycin, streptomycin, amikacin, tobramycin and monomycin used as veterinary preparations along with GM, were not recorded until their concentration of 10 000 ng/ml. This fact indicated high specificity of the analysis and the possibility of using both variants of ELISA for selective detection of GM in samples. Interestingly, that ELISA with antibodies to BSA-GM and the immobilized conjugate GM-ovalbumin showed cross-reactivity with other aminoglycoside antibiotics (kanamycin, streptomycin and neomycin) below 1% (12). Earlier, to induce highly specific antibodies to GM, it was recommended by M.B. Lui et al. (13) a special procedure when firstly the conjugate with BSA in complete Freund's adjuvant is injected into the rabbit’s paws pads, and then repeatedly - GM conjugates with red blood cells intravenously. The authors’ findings suggest no need in such extremely traumatic procedure usually hardly endured by animals.
Thus, both GM conjugates synthesized for immunization purposes induced the production of highly specific antibodies during a common procedure. Abroad, the control of GM residues in animal products is performed by available diagnostic kits in a variety of formats – from rapid assessment tests such as ELISA-card (EZ-Screen: Gentamicin, “Environmental Diagnostics, Inc.”, USA), ELISA-tube test (LacTek Milk Gentamicin Screening Kit, “Idetek, Inc.”, USA), ELISA-probe (Cite Probe Gentamicin Milk Test, “IDEXX Corporation”, USA) up to traditional analysis such as ELISA-wells (Signal Gentamicin Detection Test, “Smith-Kline Animal Health Products”, USA; AGRI-SCREEN, “Neogen Corporation”, USA; Assay kits, “Techna”, Italy). Reagents obtained in this study can be used to create such test systems in our country as well.
Indirect competitive analysis based on both types of antibodies showed stable functioning during normal oscillations of external factors. The analysis of calibration graphs obtained under intermediate precision daily or at intervals of 1-2 days suggests that analytical parameters (n = 10) had a relative standard deviation less than 0,05.
During the analysis with GM solutions in 0,15 M phosphate-saline buffer (pH 7,5) containing Na2HPO4 (0,01 M), NaCl (0,14 M) and Tween 20 (0,05%) ( PBST), as well as in milk dilute 3 times with this buffer, the calibration graphs were almost identical (Fig. 2). The analysis of 19 samples of pasteurized and sterilized milk using the test system based on antibodies to BSA-GM(100) (measuring GM within the range from 1 to 100 ng/ml) revealed five samples with GM contamination from 11 to 67 mg/kg. One of seven samples of milk powder was found to be GM- positive (GM content - 30 mg/kg or 3 mg/kg reconstituted milk). Both test systems can be recommended for industrial control of milk, because the test sensitivity of 3 and 30 mg/kg ensures the detection of MCL 200 ug/kg or 0,2 mg/kg of product (14) and allows analyzing dozens of samples during 3,5-4,0 hours.
To extract GM from animal tissues, S.A. Brown et al. (15) suggested homogenizing in buffer solutions or soaking in sodium hydroxide for 20 minutes at 70 oC. Findings of this study indicate that GM can be determined in extracts of animal tissues prepared by adding to the portion of pre-dried homogenate of an equal amount (weight / volume) of the acetonitrile-water mixture at a ratio of 84:16. During the analysis based on antibodies to BSA-GM(100) using water-acetonitrile mixture and water-acetonitrile extract of chicken muscle tissue at a 10-fold dilution with PBST, calibration graphs were almost similar to those obtained for PBST and milk diluted with PBST (measuring GM within the range from 1 to 100 ng/ml) (Fig. 2). Under these conditions, the lower limits of detecting GM in meat using the two proposed test systems were 10 and 100 mg/kg. Current domestic regulations for permitted content of GM residues in meat and liver of cattle, sheep, pigs and poultry (0,1 mg/kg) and kidney (2,0 mg/kg) (14) exceed sensitivity of the developed ELISA test systems in 1 - 3 orders of magnitude. As a rule, GM is not added to feeds, but if necessary, these test systems allow the analysis of water-acetonitrile extracts of feeds with the lower limit of detection of 5 mg/kg.
So, the developed variants of indirect competitive enzyme-linked immunosorbent assay (ELISA) were found to provide highly sensitive and selective determination of gentamicin, and they can be used to monitor the antibiotic residues in livestock products.
REFERENCES
1. Klenova I.F. and Yaremenko N.A., Veterinarnye preparaty v Rossii: Spravochnik (Veterinary Medications in Russia: Reference Book), Moscow, 2001.
2. Spravochnik veterinarnykh preparatov (Reference Book of Veterinary Medications), Pankovets E.A., Ed., Minsk, 1996.
3. Phaneuf D., Francke E. and Neu H.C., Rapid, Reproducible Enzyme Immunoassay for Gentamicin, J. Clin. Microbiol., 1980, vol. 11, no. 3, pp. 266-269.
4. Ara J., Gans Z., Sweeney R. and Wolf B., Dot-ELISA for the Rapid Detection of Gentamicin in Milk, J. Clin. Lab. Anal., 1995, vol. 9, no. 5, pp. 320-324.
5. Haasnoot W. and Verheijen R., A Direct (Non-Competitive) Immunoassay for Gentamicin Residues with an Optical Biosensor, Food Agricult. Immunol., 2001, vol. 13, no. 2, pp. 131-134.
6. Nakane P.K. and Kawaoi A., Peroxidase-Labeled Antibody. A New Method of Conjugation, J. Histochem. Cytochem., 1974, vol. 22, no. 2, pp. 1084-1091.
7. Hermanson G.T., Bioconjugate Techniques, San Diego—New York—Boston—London—Sydney—Tokyo—Toronto, 1996.
8. Burkin A.A., Kononenko G.P. and Burkin M.A., Methods of Sanitary Control of Livestock Products. I: Ensymoimmunoassay (ELISA) of Tetracyclines, S.-kh. biol., 2010, no. 4, pp. 110-117.
9. Burkin A.A. and Burkin M.A., Obtaining Antibodies to 1,4-Dyhydropyridine Calcium Channel Antagonists, Prikladnaya biokhimiya i mikrobiologiya, 2008, vol. 44, no. 3, pp. 357-361.
10. Burkin A.A. and Burkin M.A., Enzyme Immunoassay of Clofelin, Sudebno-meditsinskaya ekspertiza, 2007, no. 4, pp. 30-32.
11. Clark E.G.C., Isolation and Identification of Drugs, London: The Pharmaceutical Press, 1986.
12. Kolosova A.Yu., Blintsov A.N., Samsonova Zh.V. and Egorov A.M., Development of an Enzyme-Linked Immunosorbent Assay of Gentamicin in Human Blood Serum, Antibiotiki i khimioterapiya, 1998, no. 2, pp. 2: 9-13.
13. Lui M.B., Blackstock R. and Hyde R.M., A Rapid Method to Produce Anti-Gentamicin Antibody, J. Antibiot., Tokyo, 1981, vol. 34, no. 7, pp. 898-901.
14. Sanitary Rules and Regulations SanPin 2.3.2.1078-01 Hygienic Requirements for Safety and Nutritional Valueof Foodstuff, Moscow, 2002.
15. Brown S.A., Newkork D.R., Hunter R.P., Smith G.G. and Sugimoto K., Extraction Methods for Quantitation of Gentamicin Residues from Tissues using Fluorescence Polarization Immunoassay, J. Assoc. Off. Anal. Chem., 1990, vol. 73, no. 3, pp. 479-483.
1I.I. Mechnikov All-Russia Research and Development Institute of Vaccines and Sera, RAMS, Moscow 105064, Russia |
Received November 8, 2010 |