УДК 591.05:577.161.3
ABOUT PARTICIPATION OF α-TOCOPHERILQUINONE IN GLUCOSE FERMENTATION BY Saccharomyces cerevisiae
V.I. Dudin
The author studies the effect of α-tocopherilquinone on fermentation processes in cultures of dry freshly-prepared and long-term keeping baker's yeast Saccharomyces cerevisiae in incomplete medium with D-glucose or sodium pyruvate. It was established, that α-tocopherilqui-none takes an active part in fermentation of the second stage of glycolysis. The utilization of exogenous pyruvate reduces in experiments with dry fresh baker’s yeast, but raises in experiments with yeast after its keeping.
Key words: dried baker`s yeast Saccharomyces cerevisiae, glucose, acetic aldehyde, α-tocopherolquinone, 3-deoxy-α-tocopherolquinone, pyruvic acid, fermentation.
α-tokopherilquinone is a product of free radical oxidation of vitamin E in the body. It has no specific transferring agent, but there was found a protein with a molecular mass of 40 kDa, which associates with α-tokopherilqinone in the liver (1). The initial substance for biosynthesis of quinone - α-tokopherol – is associated with another protein. a-tokopherilquinon is often regarded as an active metabolite of vitamin E (2). It was shown, that intramuscular or oral introduction of exogenous α-tokopherilquinone inhibits growth of animals, which may be affected by α-tokopherilquinone shunting into the metabolic chain between NADH dehydrogenase and molecular oxygen (3).
The yeast Saccharomyces cerevisiae have some properties of eukaryotic cells and is a well studied genetic system, which makes it rather the perfect model for studying regulatory systems in animals and humans than the classical prokaryotic subject of cell biology - Escherichia coli (4, 5) .
The purpose of our work was studying the effect of α-tokopherilquinone on fermentation processes in the baker's yeast Saccharomyces cerevisiae.
Methods. In each experiment authors used the commercial baker's yeast Saccharomyces cerevisiae (Strain S11M, XTP) («Lesaffre», France) - the dry freshly-prepared yeast and kept in a refrigerator at +4 °С in open packaging within a year. Experiments were performed in 3 replications. D,L-α-tokopherilquinone was obtained by oxidation of D,L-α-tokopherole with nitric acid (6), with the consequent purification in a column of silica gel under the control of high-performance liquid chromatography (HPLC) (7).
In experiment 1, D-glucose (4g) was dissolved in water (200 ml), poured of 100 ml into two flasks (each of 300 ml volume); into the 1st flask α-tokopherilquinone (10 ml in 10 ml water and 150 mg Tween-80) was added, into the 2nd (control) - the same quantity in water and Tween-80. 0.75 g yeast was placed into each flask, intensively stirred in a water bath with shaker (model 357 by «Olpon», Poland) (30 °С, 48 h); the test samples were taken by a pre-established scheme: the first sample - in 12 minutes after adding yeast, the following - after 3,5, 7, 14, 24, 36 and 48 h.
In the experiment 2, sodium pyruvate (1 g) was dissolved in water (200) ml and poured of 100 ml in two flasks (each of 300 ml volume); into the 1st flask the same quantity of a-tokopherilquinone was added (control – without α-tokopherilquinone) and 1 g yeast placed into the both flasks. The flasks were incubated in a water bath with shaker, and 1 day later sodium pyruvate (250 mg) was added into the both flasks; the test samples of 5 ml were taken (after 12 min, 3,5, 7, 14, 24, 36 and 48 h). HPO3 (1,25 ml 10 % solution) was added to the samples as a precipitant of protein and centrifuged in the refrigerated centrifuge (model K 24 by «Janetzki», Germany) with an angular rotor (12х11 ml, 28°) at 10 000 g and +4 °C.
Concentration of pyruvic acid was evaluated by its ability to form 2,4-dinitrophenylhydrazones, which were purified by reverse extraction from toluene solution into sodium carbonate (Na2CO3) aqueous solution and converted into the aci-form by addition of sodium hydroxide solution (8). Concentration of a-tokopherilquinone was measured by direct-phase HPLC (the chromatograph Milihrom, Russia) with steel column 120 х 2 mm, filled with silasorb-600 (by «Lachema», Czech Republic), particle diameter - 6 mm, eluent - mixture of hexanes: sulfuric ether: methanol (89:10:1) (7).
Concentration of hexoses was found by the method based upon their dehydration to formaldehyde, whose concentration was measured in its reaction with chromotropic acid (9). Acetaldehyde (as 2,4-dinitrophenylhydrazone) was isolated from toluene solution remaining after extraction of 2,4-dinitrophenylhydrazone of pyruvic acid. The solution was evaporated and purified by double preparative thin- layer chromatography with silica gel L («Lachema», Czech Republic) and benzene. Hydrazone was extracted with methanol. The concentration of aldehyde was determined using the spectral colorimeter Specol-11 («Carl Zeiss», Germany) at 350 nm.
Authors used the reagents of domestic production with qualifications "chemically pure" or "analytical grade". Statistical processing of data was performed upon the common principles (10) using Microsoft software (Microsoft Excel).
Results. In the version with freshly prepared yeast (experiment 1), α-tokopherilqinone induced the noticeable decrease in concentration of pyruvic acid in incubation medium (Fig. 1, A, a), in experiment 2 - the sharp decrease in the rate of source material utilization in comparison with control ( See Fig. 1, A, b). In experiment 1, the difference between averages was +20,2±0,27 % (Р = 0,014), in experiment 2 - +34,6±8,80 % (Р = 0,054).
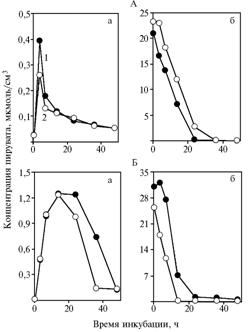 |
Fig. 1. Concentration of pyruvate in incubation medium with glucose (a) and sodium pyruvate (b) in the experiment with dry freshly-prepared (A) and subjected to long-term keeping (Б) yeast Saccharomyces cerevisiae: Explanation: |
Long-term keeping of yeast significantly affected its ability to ferment glucose at an intensive stirring of incubation medium. After adding α-tokopherilquinone (see Fig. 1, B, a) and incubation for 14 h, the decrease in concentration of pyruvate was observed (+17,2±0,5 %; Р = 0,007). Concentration of substance in medium between 36 and 48 hours of incubation was minimal and did not change. These data indicate the acceleration of fermentation, induced by α-tokopherilquinone. In the experiment with pyruvate the same acceleration of metabolism was found (see Fig. 1, B, b) (+45,2±5,4 %, Р = 0,025).
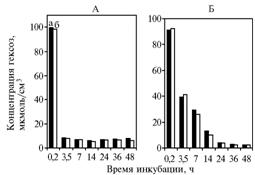
The similar dynamics of hexoses content was found in experiment 1 (version with freshly-prepared yeast) and in control: it decreased rapidly by 3.5 h without any further significant changes (Fig. 2, A). For the long-term kept yeast, concentration of hexoses decreased gradually, indicating the loss of activity due to the lon-term keeping (see Fig. 2, B).
The absence of any significant difference in fermentation of glucose between control and experiment demonstrates the effect of α-tokopherilquinone, which accelerated the process during the second stage.
Sulfhydryl groups, such as cys-149 in active center of phosforylating glycero-aldehyde-3-phosphate dehydrogenase (GAPDH, CF 1.2.1.12), are the most probable target for oxidants attacks. GAPDH oxidation with hydrogen peroxide or enzyme deterioration result in formation of inactive "oxidized" enzyme, which doesn’t perform the absorption at 360 nm.
The restoration, for example, after applying glutathione (11), reverses the initial activity and absorption spectrum. Mild oxidation of SH-groups cys-149 with hydrogen peroxide (100 mkMol) initiates synthesis of sulphen acid (12) and, consequently, the decrease in dehydrogenase activity and emergence of acylphosfatase activity, which ultimately accelerates glycolysis with a decrease of ATP release.
|
Fig. 2. Concentration of hexoses in incubation medium with glucose in the experiment with dry freshly-prepared (A) and subjected to long-term keeping (Б) yeast Saccharomyces cerevisiae: Explanation: |
Oxidation of sulfhydryl groups in GAPDH active centers may occur via interaction with nitric oxide (13). NO or its derivatives interact with thiol groups to form nitrosothiols. Nitrosation of enzymes like GAPDH inhibits their catalytic activity. Acceleration of glycolysis also occurs at the replacement of phosphate with arsenate (14).
During this process, triose phosphate oxidation is not accompanied with esterification of inorganic phosphate. Like phosphate, arsenate forms an intermediate with phosphoglyceric aldehyde, but, unlike 1,3-diphosphoglyceric acid, the arsenate-containing oxidation product rapidly dissolves in aqueous solution with no need in acceptor for arsenate. 1,3-diphosphoglyceric acid is unstable in aqueous solutions (half-time - 27 minutes), but its rate of decomposition is still small, therefore, the specific acceptor (ADP) is required to remove phosphate from carboxyl. When glycolysis occurs in bypass to phosphoglycerate kinaze reaction, it accelerates (12).
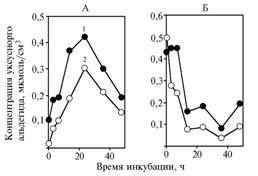 |
Fig. 3. Concentration of acetaldehyde in incubation medium with glucose (A) and sodium pyruvate (Б) in the experiment with yeast Saccharomyces cerevisiae subjected to long-term keeping: Explanation: |
In both experiments 1 and 2 (the variant with long-term kept yeast), adding α-tokopherilquinone to incubation medium induced the significant decrease in concentration of acetaldehyde (Fig. 3) (+42,0±1,7 % , Р = 0,0003 and +31,5±0,35 % , Р = 0,002, respectively): acetaldehyde content in medium was about 1% of unused pyruvate.
Centrifugation of samples incubated with glucose substrate revealed transfer of a-tokopherilquinone into yeast cells (Fig. 4, A), which was slightly accelerating at the decrease in concentration of the substance in supernatant.
Using pyruvate as substrate, the found differences were much more contrasting (see Fig. 4, B) have maintained the trend of observed alterations, as well as in the variant with long-term kept yeast (data not shown).
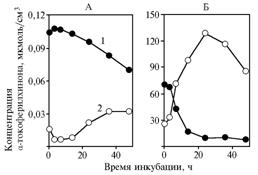 |
Fig. 4. Concentration of a-tokopherilquinone in incubation medium (1) and in precipitate (2) in experiments with glucose (A) and sodium pyruvate (Б) as substrates for yeast Saccharomyces cerevisiae Explanation: |
α-tokopherilquinone affects the studied processes, probably, through its transformation into a substance with a lower chromatographic mobility (the volume of eluent bringing a peak of substance into cuvette - 398 ml against 900 ml).
The alterations in concentration of this a-tokopherilquinone derivative depending on time of incubation (Fig. 5) follow the trends of the initial substance concentration in yeast centrifugates (for pyruvate - r = 0,97, P <0,05; for glucose - r = 0,95, P <0,05). Synthesis of the presumed active agent occurs with participation of cell structures, while an average concentration of a-tokopherilquinone in yeast precipitate is largely dependent on substrate (for pyruvate - 0,132 mkMol/sm3; for glucose - 0,017 mkMol/sm3).
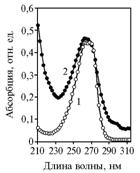
The "leakage" of a-tokopherilquinone in experiment 1 could originate from its destruction at the fermentation, or transformation into the unknown substance with UV-absorption spectrum close to α-tokopherilquinone (Fig. 6), which most likely corresponds to the non-polar α-tokopherilquinone - 3-deoxy-α-tocopheryl-p-quinone.
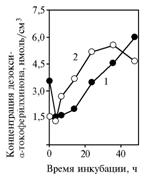 |
Fig. 5. Concentration of non-polar a-tokopherilquinone in cells of yeast Saccharomyces cerevisiae (1) in experiments with glucose (A) and sodium pyruvate (Б) as incubation medium substrates. Explanation: |
For example, 3-deoxy-α-tocopheryl-p-quinone is found in Butyrivibrio fibrisolvens extract as an alternative to authentic a-tokopherilquinone, and is synthesized from it; the partially purified enzyme isolated from bacteria catalyzes restoration of a-tokopherilquinone into the corresponding quinol at NADH presence with a stoichiometry of 1:1 (15).
In intact yeast cells, we haven’t found any authentic or non-polar a-tokopherilquinone. Nevertheless, the fact of non-polar quinone biosynthesis from the added one, indicates that yeast retain the ability to metabolize a-tokopherilquinone from natural substrates, particularly, in early stages of aerobic decomposition of silos, which occurs due to yeast (16) .
Thus, a-tokopherilquinone influence on fermentation processes at stages after formation of phosphotrioses is manifested by different rate of metabolization of exogenous pyruvate in dry freshly-prepared yeast Saccharomyces cerevisiae and in the long-term exposed to air at 4 °С.
|
Fig. 6. UV-absorption spectrums in a stopped flow of eluent for an authentic a-tokopherilquinone (1) and its derivative synthesized in yeast Saccharomyces cerevisiae (2). Explanation: |
In other lots of yeast, the response of fresh and long-term kept in refrigerator yeast to adding D,L-α-tokopherilquinone was significantly less contrasting (data not shown). Consequently, the level of α-tokopherilquinone influence on glycolysis depends on the nature of metabolism in yeast (or animal) cell, particularly on the competitive relationship between NAD and NADH in dehydrogenase reactions (isocitrate dehydrogenase, alcohol dehydrogenase reactions).
REFERENCES
1. Arita M., Sato Y., Arai F. e.a., Binding of A-tocopherylquinone, an Oxidized Form of A-tocopherol to Glutathione-S-transferase in the Liver Cytosol, FEBS Lett., 1998, vol. 436(3), pp. 424-426.
2. Donchenko G.V., Biokhimiya ubikhinona (Biochemistry of Ubiquinone), Kiev, 1988.
3. Dudin V.I., Grischuk S.V. and Sakovtseva T.V., About the Participation of A-tokopherylquinone and Ubiquinone in the Regulation of Growth in Pigs and Poultry, S.-ch. Biol., 2008, no. 6, pp. 53-58.
4. Berri D., Biologiya drozhzhej (Biology of Yeast), Мoscow, 1985.
5. Zhvyagil’skaya R.A. and Kotel’nikova A.V., Struktura i funktsionalnaya aktivnost’ drozhzhevykh mitokhondrij (Structure and Functional Activity of Mitochondria in Yeast), Moscow, 1991, vol.36.
6. Rao G.H.R., Keich T.P. and W h i t e J.G., Preparation, Separation and Characterization of Vitamin E Quinone, J. Chromat., 1980, vol. 196, pp. 506-511.
7. Dudin V.I., Biochimiya vitamina E i svyazannykh s nim biologicheski avtivnykh veschestv (Biochemistry of Vitamin E and the Related Biologically Active Substances), Moscow, 2004.
8. Petrun’kina A.M., Prakticheskaya biohimiya (Practical Biochemistry), Leningrad, 1961, pp. 384-390.
9. Korenman I.M., Metody opredeleniya organicheskikh soedinenij (Methods for Determination of Organic Compounds), Мoscow, 1970, pp. 65-66.
10. Merkur’eva E.K., Biometriya v zhivotnovodstve (Biometry in Animal Husbandry), Мoscow, 1964.
11. Racker A., Bioenergeticheskiye mekhanizmy (Bioenergetic Mechanizms), Мoscow, 1967.
12. Dan’shina P.V., Shmahl’gauzen E.V. and Arutyunov D.Yu., Acceleration of Glycolisis by Non-Phosphorylative and the Oxidized Phosphorylative Glyceraldehide-3-phosphate Degidrogenases., Biochimiya, 2003, vol. 68(5), pp. 725-733.
13. Asahi M., Fujii J., Suzuki K. e.a., Inactivation of Glutathione Peroxidase by Nitric Oxide. Implication for Cytotoxicity, J. Biol. Chem., 1995, vol. 270(36), pp. 21035-21039.
14. White A., Hendler F., Smit A. e.a., Osnovy biohimii (Basics of Biochemistry), Moscow, 1981, vol.2.
15. Hughes P.E. and Tove S.B., Identification Deoxy-α-tocopherolquinol as Another Endogenous Electron Donor for Biohydrogenation, J. Biol. Chem., 1980, vol. 255(24), pp. 11802-11806.
16. Mc. Donald P., Biokhimiya silosa (Biochemistry of Silage), Moscow, 1985.
Scientific Research Institute of Physiology, Biochemistry and Nutrition of Farm Animals, |
Received April 15, 2009
|