doi: 10.15389/agrobiology.2012.1.105eng
УДК 635.656:631.559.2:631.522/.524:581.138.1
SELECTION OF PEA ON RISING OF NITROGEN FIXATION WITH THE USE OF SYMBIOTIC MUTANTS
K.K. Sidorova1, A.V. Goncharova2, P.L. Goncharov2, V.K. Shumnyi1
The technology was developed for use of supernodulating mutants in pea selection for rising symbiosis efficiency. The method is based on interaction within one genotype of both recessive and dominant alleles of different sym-genes — nod4 and Nod 5, control the super- and hypernodulation. The possibility was established for simultaneous selection on rising of nitrogen fixation and productivity of macrosymbiont.
Keywords: Pisum sativum, cultivar, supernodulating mutant, nitrogen fixation, recurrent selection, productivity.
Pea is a major leguminous crop widely grown in our country as source of food and forage plant protein. Like other legumes, it has the unique ability to symbiosis with nodule bacteria and fixation of atmospheric molecular nitrogen by restoring it to ammonia. In a plant-rhizobial symbiosis, a plant acts as a source of energy whose photosynthesis products are spent on both own development, the growth of root nodules and nitrogen fixation.
Breeding work on pea for a long time was focused on improving the features of above-ground organs (stem, leaves, flowers, pods, seeds), shortening the growing season, increasing the protein content, etc. Genetics of symbiotic features aroused only in 1980s (1-4) being continued today in active studies of genetic, breeding and agro-ecological aspects of symbiotic nitrogen fixation (5-11).
The study of mutant pea has revealed in its genome numerous genes encoding symbiotic features - nodulation and nitrogen fixation. Macrosymbiont was postulated to play a key role in genetic control of symbiotic nitrogen fixation, since it is responsible for a plant’s ability to control the symbiosis with Rhizobium, the number of root nodules and their efficiency, activity of nitrogen fixation determined by a nitrogenase enzyme activity, the period of active nitrogen fixation. The efficiency of symbiosis can be influenced by different strains of nodule bacteria as well (12).
Super-nodulating mutants with increased nodulation and active nitrogen fixation are of interest in terms of selection. However, such mutants have a flaw of low productivity. In experiments of foreign researchers, super-nodulating soybean mutants produced the yield of seeds on average 25% lower than varieties with normal nodulation, although a significant positive aftereffect of the mutant genotypes was observed in following serial species of crop rotation (oats and barley) (13).
Seven mutant lines created by the authors were identified as carriers of nod-genes with established location in chromosomes. Earlier, the expression of super-nodule genes nod4 nod3 depending on the genotypic environment was studied in field experiments and in greenhouse, which revealed the prospects for using such mutants as donors in breeding pea to improve nitrogen fixation (10). It was found that hyper-nodulation is controlled by a dominant gene Nod5 located in the 3rd linkage group (14, 15).
The purpose of this research was to develop a technique for using symbiotic hyper- and super-nodulating pea mutants in breeding work aimed at increased nitrogen fixation.
Technique. The studies were conducted in field and in pot experiments (greenhouse) (2003-2011, Novosibirskaya oblast’) upon two varieties of forage pea - Druzhnaya and Novosibirskaya 1, and a donor of improved symbiotic traits – the mutant cultivar K301 induced from cv Ramonsky 77. In a greenhouse, plants were grown on a standard background of mineral nutrition (20% full rate of nitrogen was introduced in early period of growth). The seedlings were inoculated with strain 250a Rhizobium leguminosarum obtained from the collection of the All-Russia Research and Development Institute of Agricultural Microbiology (St. Petersburg-Pushkin).
Each variety was subject to reciprocal crosses with the super-nodulating mutant. Plants with super-nodulation were isolated in F2, individually harvested and used as a basis for creation of individual lines. In F3-F7, a recurrent selection of productive lines with super nodulation was performed. For each variety in F7 the selection of individual lines was carried out, as well as individual-group selection of lines by their productivity and efficiency of nitrogen fixation (11).
The number of root nodules and nitrogen fixation activity were determined in 10-15 plants from each line in the beginning of flowering using acetylene method (16) on the gas chromatograph devise Tsvet 500 (Russia). During maturation, 10-15 plants from the same plots were evaluated for productivity characteristics: plant height, number of pods, number and weight of seeds, duration of a growing season.
Root biomass and nitrogen content in it were determined according to a conventional method (calculation at a thickness of growing 1,8 million plants / ha).
Statistical processing of data was carried out according to B.A. Dospekhov (17).
Results. The varieties of pea used in the experiment, Druzhnaya and Novosibirskaya 1, were created by K.K. Sidorova, A.V. Goncharova, P.L. Goncharov et. al. These cultivars produce high yields of forage green mass and grain, and they also manifest hyper-nodulation labeled by the gene Nod5. The plants develop large nodules mainly in the upper part of the root along with the nitrogen fixation activity exceeding the average in super-nodulating varieties. The mutant form K301 has a pronounced nodulation with small nodules formed over the whole root during a longer period of time than in Druzhnaya and Novosibirskaya 1. In K301, a recessive mutation was detected and designated by the authors as nod4 with the gene located in the 5th linkage group (18).
Super-nodulation is a recessive trait, so individuals with a modified root were isolated in F2. In following generations, no splitting by this trait was observed. In F3-F7 generations of these recurrent lines, recombinogenes was the major mechanism of morphogenetic processes.
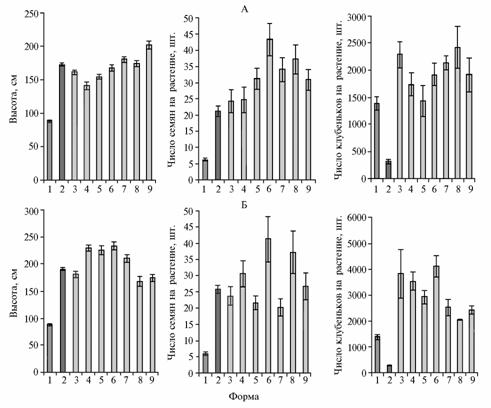
The super-nodulating mutant significantly yielded in plant height and grain productivity to cv Druzhnaya and its recurrent lines (Fig. 1, A). These lines, in turn, exhibited similar or somewhat higher indices compared with cultivars.
|
Fig. 1. Plant height, number of seeds and nodules per plant in Pisum sativum L. forage cultivars Druzhnaya (A), Novosibirskaya 1 (Б) and in their recurrent lines obtained by crossing with the super-nodulating mutant K301 induced from cv Ramonsky 77: 1 — mutant, 2 — variety, 3-9 — recurrent lines F6, resulting from the cross of mutant with a corresponding variety (field experiment, Novosibirskaya oblast’, 2009). Denotations: |
|
Fig. 2. Roots of Pisum sativum L. forage variety Novosibirskaya 1 (Nod5) (a), super-nodulating mutant К301 (nod4) (б) and recurrent line К727a with ultra-super-nodulation (nod4 Nod5) (в) (greenhouse, 2008).
|
Similar results were obtained in experiments with cv Novosibirskaya 1 (Fig. 1, B). This tall-growing variety more than twice exceeds the super-nodulating mutant by plant height. Among the recurring lines, there was isolated one variety taller than the cultivar and three lines with plant height similar to Novosibirskaya 1. Three lines developed the increased seed productivity.
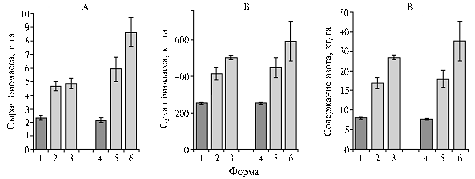
All the recurrent lines exhibited an outstanding nodulation: number of nodules on their roots was much greater than in both cultivars and the mutant (Fig. 2). The intensity of nitrogen fixation in the lines exceeded that of varieties (Table).
For a long time it was thought that it’s impossible to improve productivity and nitrogen fixation at the same time, because these processes compete for one energy source - the products of photosynthesis. However, the authors’ findings suggest a contrary assertion.
Nitrogen fixation activity in forage pea forms of Pisum sativum L. (greenhouse, 2008) |
|
Mutant / variety / recurrent line |
Nitrogenase activity, nmol С2Н4/(plant · h)-1 |
Super-nodulating mutant K301 |
1242 |
Cv Druzhnaya |
424 |
Cv Novosibirskaya 1 |
463 |
K301 × cv Druzhnaya |
1123-1226 |
Cv Druzhnaya × K301 |
2312-2380 |
K301 × cv Novosibirskaya 1 |
1235-2107 |
Cv Novosibirskaya 1 × K301 |
2399-2702 |
|
Fig. 3. Accumulation of wet (A) and dry (Б) biomass of roots, as well as nitrogen content (В) in roots of Pisum sativum L. forage cultivars and their recurrent lines obtained by crossing with the super-nodulating mutant K301 induced from cv Ramonsky 77: 1 — cv Druzhnaya; 2, 3 — recurrent lines resulting from cv Druzhnaya, 4 – cv Novosibirskaya 1; 5, 6 – recurrent lines resulting from cv Novosibirskaya 1 (greenhouse, 2009). Denotations: |
|
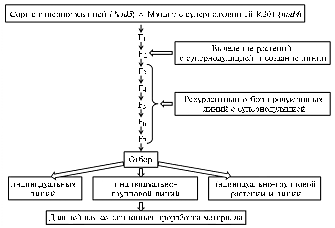
Fig. 4. A proposed scheme for selection of pea with improved nitrogen fixation using symbiotic mutants Denotations:(from top to bottom) Isolation of super-nodulating plants and creation of lines Recurrent selection of productive lines with super-nodulation Selection Subsequent selection work on the material |
Legumes are good predecessor for serial rotation crops. The new created recurrent lines exceeded cv Druzhnaya and cv Novosibirskaya 1 by weight of root biomass and nitrogen content in roots. Upon the results of multiyear studies of pea genetics, symbiosis and selection, a scheme for selection of pea with improved nitrogen fixation was developed (Fig. 4).
Thus, the combination of dominant and recessive alleles of different symbiotic genes allows to obtain forage pea forms whose seed productivity is comparable to food varieties and much better accumulation in soil of a root biomass with high nitrogen content. The use of applied genetics and selection can modify nitrogen supply of plants through a symbiotic complex, which is a quite important feature in terms of ecology and energy-saving.
REFERENCES
1. Jacobsen E. and Nijdam H.A., A Mutant Showing Efficient Nodulation in the Presence of Nitrate, Pisum Newslet., 1983, vol. 15, pp. 31-32.
2. Kneen B.E. and La Rue T.A., Induced Symbiosis Mutants of Pea (Pisum sativum) and Sweet Clover (Melilotus alba annual), Plant Sci., 1988, vol. 58, pp. 177-182.
3. Duc G. and Messager A., Mutagenesis of Pea (Pisum sativum L.) and the Isolation of Mutants for Nodulation and Nitrogen Fixation, Plant Sci., 1989, vol. 60, pp. 207-213.
4. Gresshoff P.M., Molecular Genetic Analyses of Nodulation Genes in Soybean, Plant Breed. Rev., 1993, no. 11, pp. 275-318.
5. Sidorova K.K., Stolyarova S.N. and Katysheva V.B., Nitrogen-Fixing Activity in Pea Mutants, Genetika, 1987, vol. 23, no. 7, pp. 1218-1221.
6. Sidorova K.K. and Uzhintseva L.P., The Use of Mutants for Identification of Genes Encoding Symbiotic Properties in Pea, Genetika, 1992, vol. 28, no. 4, pp. 144-151.
7. Sidorova K.K. and Shumny V.K., The Study of Supernodular Mutants of Pea (Pisum sativum L.), Genetika, 1998, vol. 34, no. 10, pp. 1452-1454.
8. Sidorova K.K. and Shumny V.K., Creation and Genetic Study of a Collection of Symbiotic Mutants of the Pea (Pisum sativum L.), Genetika, 2003, vol. 39, no. 4, pp. 501-509.
9. Sidorova K.K., Nazaryuk V.M. and Klenova M.I., A Method for Evaluation of Nitrogen-Fixing Properties of Legumes Crops, RF Patent 1 Ru 2195104, Appl. 15.04.2001, Publ. 27.12.2002, Bull. ¹ 36.
10. Sidorova K.K., Shumny V.K. and Nazaryuk V.M., Simbioticheskaya azotfiksatsiya: geneticheskie, selektsionnye i jekologo-agrokhimicheskie aspekty (Symbiotic Nitrogen Fixation: Genetic, Selection and Ecology-Agrochemical Aspects), Novosibirsk, 2006.
11. Sidorova K.K., Goncharova K.K., Goncharov P.L. and Shumny V.K., The Development of Genetic and Breeding Basics of Symbiotic Nitrogen Fixation, in Mat. V s’ezda Vavilovskogo obcshestva genetikov i selektsionerov (Proc. V Congress of Vavilov Society of Geneticists and Breeders), Moscow, 2009, vol. II, p. 216.
12. Tikhonovich I.A. and Provorov N.A., Agricultural Microbiology as the Basis of Ecologically Sustainable Agriculture: Fundamental and Applied Aspects, S.-kh. biol., 2011, no. 3, pp. 3-9.
13. Bhatia C.R., Nichterlein K. and Maluszynski M., Mutations Affecting Nodulation in Grain Legumes and Their Potential in Sustainable Cropping Systems, Euphytica, 2001, vol. 120, pp. 415-432.
14. Sidorova K.K. and Shumny V.K., A New Gene for Supernodulation in Pea (Pisum sativum L.) Nod5-nod5, Dokl. RAN, 1997, vol. 353, no. 5, pp. 703-704.
15. Sidorova K.K., Shumny V.K. and Mischenko T.M., Chromosomal Location of Nodulation Gene nod5 in Pea, Dokl. RAN, 1999, vol. 367, no. 6, pp. 851-852.
16. Hardy R.W.F., Holsten R.D., Jackson E.K. and Burns R.C., The Acetylene—Ethylene Assay for N2-Fixation: Laboratory and Field Evolution, Plant Physiol., 1968, vol. 43, no. 8, pp. 1185-1207.
17. Dospekhov B.A., Metodika polevogo opyta (Methodology of Field Experiment), Moscow, 1985.
18. Sidorova K.K. and Uzhintseva L.P., Mapping of the Mutant Nod4 Gene Responsible for Overnodulation in Pea, Dokl. RAN, 1994, vol. 336, no. 6, pp. 847-849.
1Institute of Cytology and Genetics, Siberian Brunch of RAS, Novosibirsk 630090, Russia, |
Received June 20, 2011
|