doi: 10.15389/agrobiology.2012.1.60eng
УДК 633.11:631.53.011:581.142:577.152.1
ABOUT PARTICIPATION OF OXIDOREDUCTASES IN MECHANISMS OF DORMANCY AND GERMINATION IN WHEAT CORN
V.V. Rogozhin1, T.T. Kurilyuk1, T.V. Rogozhina2
The authors estimated the activity of glucose-6-phosphate dehydrogenase, isocitrate dehydrogenase (NAD+), alcohol dehydrogenase and peroxidase in wheat corn and seedlings (Triticum aestivum L.). It was established, that these dehydrogenases are required for the retention of seed viability and for the initiation of processes, related with seed germination. The peroxidase, catalyzing the reactions with participation of oxygen and hydrogen peroxide, regulates the content of these substances in dormant corn. Considerable increase of peroxidase activity during germination of corn seeds can suggest about participation of this enzyme in activation of starting mechanism of respiration.
Keywords: grains of wheat, rest, germination, enzyme, dehydrogenase, peroxidase.
A dormancy of plant seeds is a state with reduced functional activity while maintaining a high viability when seeds meet the absence of water and low temperature. After the restoration of normal conditions, intense respiratory activity arises in seeds which then start to germinate (1).
Dormancy involves physiological and biochemical processes that operate through a complex of biologically active substances providing vitality of a plant organism during its growth and development. Transition to dormancy is accompanied by a decrease in activity of biosynthetic processes and mitochondrial metabolism owing to dissociation of oxidative phosphorylation and aerobic metabolic processes (2), while the latter outpace. Intensity of anaerobic metabolism can be evaluated from the activity of enzymes glucose-6-phosphate dehydrogenase (G6PDG), isocitrate dehydrogenase (ICDG) and alcohol dehydrogenase (ADH), aerobic metabolism – peroxidase (PO).
Glucose-6-phosphate dehydrogenase is a key enzyme in the pentose phosphate pathway of carbohydrate conversion. It provides catalytic oxidation of glucose-6-phosphate in the presence of NADP+ required for lipid metabolism of fatty acids and steroids (3). At the same time, steroid glycosides can operate as antioxidants, they regulate membrane permeability and promote cell division and growth processes (4, 5). Isocitrate dehydrogenase catalyzes the limiting stage of the tricarboxylic acid cycle in mitochondrial matrix. Alcohol dehydrogenase catalyzes the reversible oxidation of aliphatic alcohols with the participation of NAD+ (6). Concentration ratio of aldehyde and alcohol reflects the intensity of anaerobic bioenergetic reactions. Reduce in this rate is accompanied by activation of catabolic processes, whereas the increase leads to a deeper dormancy. However, the role of this regulatory mechanism in seed germination needs a better study.
Peroxidase jointly with SOD and catalase operates as one antioxidant defense system preventing the damage of living organisms from reactive oxygen species (7, 8). The enzyme is capable to catalyze oxidation of various biologically active compounds (NADH, indole-3-acetic acid, ascorbic acid, flavonoids, etc.) including antioxidants (AO) - substances that can inhibit the formation of free radicals and lipid peroxidation (LPO) (9, 10).
Seed germination includes three periods (11): metabolism activation (physical swelling), preparation to the start of elongation (seed shell opens to release the embryo whose axial organs increase owing to cell growth) and growth of a seedling. Normal germination of air-dry seeds can occur in the presence of all components of the protein-synthesizing system: ribosomes, tRNAs, factors of initiation and elongation, amino acids and aminoacyl-tRNA synthases, all metabolic enzymes, heat shock proteins and their mRNAs. Wet seeds absorb lots of oxygen, which can cause oxidative damage in tissues. Reactive oxygen species (ROS: O2-, H2O2, NO·, NOCl et al.) play an important role in development of the oxidative stress (12). Accumulation of ROS in cells results in disruption of transcription and replication processes, and it also changes lipid composition of biomembranes. Superoxide radicals modify proteins, they disrupt the structure of DNA, hormones and other functionality active substances (13).
The purpose of this research – to study the activity of key oxidoreductases (glucose-6-phosphate dehydrogenase, NADP+-dependent isocitrate dehydrogenase, alcohol dehydrogenase and peroxidase) during a storage and germination of wheat weevils along with assessment of their role in control of dormancy and germination.
Technique. The study was performed on wheat grain (Triticum aestivum L.) cv Prilenskaya 19. The weevils were placed in distilled water for 24 h and then germinated on filter paper in Petri dishes at 23 °C in the light for 7 days wetting with distilled water (10 ml per Petri dish). The number of seeds per one cup - 100 pcs., test replication– 4-fold. Viability of the weevils was determined using tetrazolium test (14).
To analyze the reaction products of thiobarbituric acid (TBA) and the content of antioxidants, seeds or seedlings (1 g wet weight) were homogenized in a porcelain mortar with 3 ml 50% ethanol solution. The homogenate was centrifuged for 10 min at 7000 g. The content of malondialdehyde (MDA) was determined using the revised method based on reaction with thiobarbituric acid (λ = 532 nm, e = 155 mM-1·sm-1) (10). 0,5 ml supernatant was added with 0,5 ml 1% Triton X-100 solution, then 0,2 ml 0,6 M HCl and then 0,8 ml 0,06 M TBA. The mixture was heated in a boiling water bath for10 min and then cooled at 15 °C for 30 min. A color was stabilized by 0,2 ml 5 mM Trilon B and 5-10 ml 96% ethanol. Control samples were treated with the same solutions except for TBA.
Antioxidants were analyzed according to the adopted method (15). 0,2 ml supernatant was added consecutively with 0,2 ml 0,5% o-phenanthroline dissolved in 96% ethanol and 0,2 ml 0,2% FeCl3 in 96% ethanol. Then the volume of the solution was adjusted to 3 ml with 96% ethanol and left in the dark for 10 minutes. Antioxidant content was determined from the calibration curve designed for quercetin.
To determine the activity of peroxidase and other enzymes in seeds or seedlings, a sample (1 g) was homogenized in a porcelain mortar with 3 ml 0,1 M sodium phosphate buffer (pH 7,0). The homogenate was centrifuged for 10 min at 7000 g. Peroxidase activity was assessed from the initial rate of oxidation of o-dianisidine by hydrogen peroxide at 22 ° C. To do this, 2,1 ml 0,1 M sodium phosphate buffer (pH 7,0) was added with 0,2 ml supernatant and 0,1 ml 0,43 mM o-dianisidine solution in 96% ethanol. The reaction was initiated by introducing 0,1 ml 16 mM hydrogen peroxide. After a rapid mixing, the increase in optical absorption of the solution (l = 460 nm, e = 30 mM-1·sm-1) during the oxidation of o-dianisidine was recorded (16). The accepted unit of enzyme activity was a quantity of o-dianisidine (umol) oxidized for 1 min calculated per 1 g dry weight of a sample. Activity of alcohol dehydrogenase, NADP+-isocitrate dehydrogenase and glucose-6-phosphate dehydrogenase were determined according to earlier developed methods (17-19).
The activity of ADH was evaluated from the rate of ethanol oxidation and formation of NADH (l= 340 nm, e = 6,22 mM-1·sm-1). 2,2 ml 0,1 M glycine-NaOH buffer (pH = 10) was added with 0,1 ml 36 mM NAD+ solution and 0,1 ml 0,41 M ethanol solution. The reaction was initiated by introducing 0,1 ml of the supernatant. The accepted unit of enzyme activity was a quantity of NAD+ (umol) restored for 1 minute during the process of ethanol oxidation.
The activity of glucose-6-phosphate dehydrogenase was determined at consecutive adding in the cuvette 2,2 ml 0,01 M Tris-HCl buffer (pH 7,4), 0,1 ml 0,1 M MgCl2 solution, 0,1 ml 0,025 M NADP+ solution and 0,1 ml 0,05 M solution of glucose-6-phosphate. The reaction was initiated by introducing 0,1 ml supernatant. The accepted unit of enzyme activity was a quantity of NADPH (umol) reduced during 1 minute in the process of glucose-6-phosphate oxidation.
The enzyme activity of NADP-dependent isocitrate dehydrogenase was determined from the rate of isocitrate oxidation and formation of NADPH (l = 340 nm, e = 6,22 mM-1·sm-1): 2,6 ml 0,1 M Tris-HCl buffer (pH 7,5), 0,1 ml 4 mM NADP+ solution, 0,1 ml 0,1 M MnCl2 solution and 0,1 ml 0,1 M isocitrate Na were sequentially introduced in a cuvette. The reaction was initiated by introducing 0,2 ml supernatant.
Dormant wheat grains harvested in different years (2002-2007) were analyzed to determine the activity of peroxidase and ADG, as well as other reserve catabolic and anabolic dehydrogenases - glucose-6-phosphate dehydrogenase and NADP-dependent isocitrate dehydrogenase.
Spectrophotometry was carried out on a double-beam spectrophotometer DMS 100 S (“Varian”, USA). The study was performed using NAD+, NADP+, G6PDG (“Reanal”, Hungary), ethanol purified by distillation, o-dianisidine the grade “Pur.” purified by sublimation in a vacuum, hydrogen peroxide (30% aqueous solution) and antioxidants the grade “Puriss. spec.”.
Statistical processing of data was performed by biometric calculations (20). The presented data show mean values and their deviations.
Results. On the 4th-7th days of germination, MDA content in the endosperm and roots of wheat seedlings were just slightly different - respectively, 26,3-45,7 and 46,6-53,5 nmol/g dry weight; at the same time, MDA content in green cotyledons was 4-15 times higher and varied from 219 to 623 nmol/g dry weight. Antioxidant content in the endosperm amounted to 0,379-0,579, in roots - 0,650-1,980, in green cotyledons - 2,060-4,790 mg/g dry weight. Therefore, MDA content in seedlings always was inversely proportional to the content of antioxidants. At the same time, the rate of lipid peroxidation was unequal in different parts of a plant: the maximum LPO rate while the high content of antioxidants was found in green seedlings, lower rates - in roots and endosperm (Table 1)/
| 1. Dynamics of antioxidant content and malonaldehyde content in seeds and seedlings of wheat cv Prilenskaya 19 | ||||||
Time of germination, days |
Grain |
Roots |
Aboveground parts |
|||
AO |
MDA |
AO |
MDA |
AO |
MDA |
|
4th |
0,579±0,180 |
26,3±1,4 |
1,690±0,150 |
53,4±4,2 |
3,010±0,240 |
219,0±12,0 |
6th |
0,379±0,090 |
39,4±3,1 |
0,650±0,080 |
46,6±3,6 |
2,060±0,180 |
623,0±18,0 |
7th |
0,555±0,120 |
45,7±3,3 |
1,980±0,180 |
53,5±3,8 |
4,790±0,350 |
494,0±15,0 |
Note. AO – antioxidants, mg/g dry weight; MDA – malondialdehyde, nmol/g dry weight. In brackets – percentage to the initial value (on the 4th day). |
||||||
2. Activity of alcohol dehydrogenase and peroxidase in germinating and dormant viable weevils of wheat cv Prilenskaya 19 |
||
Time of germination, days |
ADH |
PO |
Control (dry) |
||
|
3,8±0,3 |
3,0±0,3 |
Germinating |
||
1st |
2,1±0,3 |
7,2±0,7 |
3rd |
0,7±0,1 |
8,5±0,8 |
Dormant viable |
||
1st |
4,3±0,4 |
2,4±0,2 |
3rd |
4,6±0,4 |
3,5±0,2 |
Note. ADG – alcohol dehydrogenase, umol / (minšг dry weight); PO — peroxidase. |
||
ADH and peroxidase neutralize the effects of LPO products. ADH catalyzes the reduction of aldehydes, peroxidase controls the content of hydrogen peroxide and AO. The latter are a substrate for the enzyme and they are capable to participate both oxidase and peroxidase reactions. Swelling and germination of the grains were accompanied by a further decrease in ADG activity relative to that in dormant grains (on the 3rd day – by more than 5,4-fold) (Table 2). In this period, the enzyme activity in non-germinated viable grains increased by 13-21%. Peroxidase activity in germinating grains increased in 2,4-2,8 times, while it remained almost unchanged in non-germinated grains.
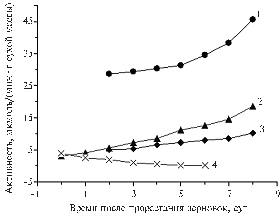 |
Activity of peroxidase in roots (1), weevils (2) and seedlings (3), as well as alcohol dehydrogenase in weevils (4) of wheat cv Prilenskaya 19 depending on time after germination Denotations: |
Therefore, ADG is necessary primarily in dormant grains, whereas peroxidase helps to maintain the viability of dormant grains being also capable to promote red-ox reactions in early germination.
The figure shows that activities of PO and ADG in dormant wheat grains are comparable. Germination of weevils was accompanied by the increase in PO activity in aboveground parts of seedlings in 1,8-2,0 times, in weevils - 4-5 times, in roots - 12-14 times. Along with it, by the 6th day, ADG activity dropped almost to a zero. Individual charts of increase in PO activity peculiar to different parts of wheat seedlings was apparently associated with different ratios of low molecular weight AO (Table 1). There was observed a correlation between PO activity and AO content in aboveground parts and roots of seedlings.
The analysis of wheat weevils stored for several years has shown a reduce in seed viability along with dehydrogenase activity, while a decrease in germination was correlated with reduce in PO activity (Table 3).
3. Oxidoreductase activity in grain of wheat cv Prilenskaya 19 depending on duration of a storage period |
||||||
Storage |
Viability, % |
Germination, % |
Activity, umol/(minšg dry weight) |
|||
ADG |
PO |
G6PDG |
ICDG |
|||
2002-2003 |
99±1 |
96±2 |
2,89 |
3,15 |
1,75 |
2,83 |
2002-2005 |
88±2 |
79±5 |
1,56 |
1,59 |
1,22 |
2,11 |
2002-2007 |
56±2 |
24±2 |
1,26 |
0,75 |
0,78 |
1,39 |
Note. ADG – alcohol dehydrogenase, PO – peroxidase, G6PDG – glucose-6-phosphate dehydrogenase, ICDG – isocitrate dehydrogenase. In brackets – percentage to the value at a 1-year storage. |
||||||
Previously, it was shown that peroxidase participates in maintaining the viability of cereal grains when they experience a forced dormancy and the lack of exogenous water at this time, because this enzyme is capable to catalyze a sequential reduction of oxygen to water in response to embryo’s demands (21 -24).
Thus, dehydrogenases including alcohol dehydrogenase are necessary primarily for maintaining the viability of dormant grains and the startup of processes related to seed germination, while peroxidase regulates the content of oxygen and hydrogen peroxide in dormant grains as it catalyzes the reactions involving these substances. During germination, the intensity of aerobic bioenergetic processes increases and the activation of oxidases occurs. The sharp advance in peroxidase activity suggests that the enzyme participates the promotion of germination triggers by initiating the reactions of free radical oxidation, which, in turn, activates lipid peroxidation and contributes to increased energy metabolism in mitochondria.
REFERENCES
1. Nikolaeva M.G., Razumova M.V. and Gladkova V.N., Spravochnik po proraschivaniuy pokoyaschikhsya semyan (Guidelines on Germination of Dormant Seeds), Leningrad, 1985.
2. Kershengolts B.M., Ethanol and Acetaldehyde in Organisms of Plants and Animals, Extended Abstract of Doctoral Dissertation, Moscow, 1991.
3. Berezov T.T. and Korovkin B.F., BIokhimiya (Biochemistry), Moscow, 2007.
4. Bogatskiy A.V., Nazarova N.Yu. and Kintya P.K., Modification of Double-Layer Lipid Membranes in Steroid Glycosides, Dokl. AN SSSR, vol. 252, no. 1, pp. 235-237.
5. Kintya P.K., Natural Steroid Bioregulators, Zhurn. Vses. khim. obschestvaim. D.I. Mendeleeva, 1988, vol. 33, no. 5, pp. 584-586.
6. Rogozhin V.V., Govorova T.P. and Kershengolts B.M., The Method of Selective Titration of Alcohol Dehydrogenase Active Sites with Chlor- and Hydroxymercuribenzoate, Bioorganicheskaya khimiya, 1988, vol. 14, no. 12, pp. 1626-1631.
7. Fridovich I., Biological Effects of the Superoxide Radical, Arch. Biochem. Biophys., 1986, vol. 247, no. 1, pp. 1-11.
8. Halliwell B., The Toxic Effects of Oxygen on Plant Tissues, in Superoxide dismutase, Oberley L.W., Ed., Boca Raton: CRC Press, 1982, vol. 1, pp. 89-123.
9. Lebedeva O.V. and Ugarova N.N., The Mechanism of Peroxidase Oxidation. Substrate-Substrate Activation in Reactions Catalyzed by Horseradish Peroxidase, Izv. RAN, Seriyakhimicheskaya, 1996, no. 1, pp. 25-32.
10. Vladimirov Yu.A. and Archakov A.I., Peroksidnoeokislenielipidovvbiologicheskikhmembranakh(Lipid Peroxidation in Biomembranes), Moscow, 1972.
11. Obrucheva N.V. and Antipova O.V., Physiology of Seed Germination, Fiziologiya rastenii, 1997, vol. 44, no. 2, pp. 287-302.
12. Zenkov N.K. and Men’shikova E.B., Activated Oxygen Metabolites in Biological Systems, Uspekhi sovremennoi biologii, 1993, vol. 113, no. 3, pp. 286-296.
13. Kogan A.Kh., Kudrin A.N., Kudrin A.N., Kakturskiy L.V. and Losev N.I., Free-Radical Peroxidation Mechanisms in Pathogenesis of Ischemia and Myocardial Infarction, Patofiziologiya i eksperimental’naya terapiya, 1992, no. 2, pp. 5-15.
14. Zhiznesposobnost’ semyan (Viability of Seeds), Firsova M.K., Ed., 1975.
15. Metody biokhimicheskogo issledovaniya rastenii (Methods of Biochemical Analysis of Plants), Ermakov A.I., Ed., 1987.
16. Lebedeva O.V., Ugarova N.N. and Berezin I.V., The Kinetic Study of Oxidation of O-Dianisidine with Hydrogen Peroxide in the Presence of Horseradish Peroxidase, Biokhimiya, 1977, vol. 42, no. 8, pp. 1372-1379.
17. Kershengolts B.M. and Rogozhin B.V., The Influence of Interaction between the Subunits of Alcohol Dehydrogenase from a Horse Liver on the Kinetics of Ethanol Oxidation, Bioorganicheskaya khimiya, 1979, vol. 44, no. 4, pp. 661-671.
18. Rogozhin V.V., Possible Mechanisms in Regulation of Activity of Glucose-6-Phosphate Dehydrogenase by Excess of Substrate and Coenzyme, Bioorganicheskaya khimiya, 1996, vol. 23, no. 8, pp. 575-579.
19. Metody biokhimicheskikh issledovanii (Methods of Biochemical Analysis), Prokhorova M.N., Ed., Leningrad, 1982.
20. Lakin T.F., Biometriya (Biometry), Moscow, 1990.
21. Rogozhin V.V., Peroksidasa kak component antioksidantnoi sistemy zhivykh organizmov (Peroxidase as a Component of Antioxidant System in Living Organisms), St. Petersburg, 2004.
22. Rogozhin V.V. and Peretolchin D.V., The Kinetics of Ascorbic Acid Oxidation in the Presence of Horseradish Peroxidase, Vest. YuUrGU, Ser. khimiya, 2010, vol. 11, no. 187, pp. 61-65.
23. Rogozhina T.V. and Rogozhin V.V., The Role of Antioxidant System Components in the Mechanisms of Seed Germination, Vest. AGAU, 2010, vol. 11, no. 73, pp. 31-38.
24. Rogozhin V.V. and Kurilyuk T.T., The Role of Peroxidase in Mechanisms of Dormancy and Germination in Cereal Crops, Izv. TSKhA, 2010, no. 4, pp. 22-31.
1Yakutsk State Agricultural Academy, Yakutsk 677002, Russia |
Received November 27, 2009 |













