doi: 10.15389/agrobiology.2012.1.66eng
УДК 633.11:575.22:631.52:[581.132+631.524.8
NET PHOTOSYNTHESIS AND EAR GRAIN CONTENT IN THE VARIETIES OF SPRING WHEAT UNDER THE INFLUENCE OF DONOR-ACCEPTOR RELATIONS
E.F. Ionov1, N.E. Ionova2, V.A. Dragavtsev3
With the use of developed graphical method for the estimation of genotypic resistance of plants to stress (factor of stress is the deletion of two upper leaves at the period of ear formation, net photosynthesis was estimated on total air-dry mass of top) from broad spectrum of spring soft wheat varieties the authors isolated the agroecotypes with optimal combination of morphological determinants, permitting to form the increased yield, that can be used for genetic improvement of attract ability of wheat ear.
Keywords: spring wheat, stress, defoliation, source-sink relation, attraction, net-weight- photosynthesis.
One of the most important targets of plant breeding is investigation and improvement of adaptive properties of cereal crops with respect to the norm of reaction to stressors (tolerance). However, resistance to abiotic and biotic stressors today becomes an extremely rare property in cultivated varieties (1). Domestication of wheat has led to a great diversity of wheat species and varieties. Wheat is a cosmopolitan among cereal crops for the variety of its forms and total area of cultivation. However, breeding and genetic improvement of commercially important traits of cereals along with growing in comfortable man-made artificial field conditions has reduced their adaptability in productivity and quality characteristics.
Adaptability of agroecotype, or the degree of its stability under a particular stressor impact, can be evaluated by reduce in total productivity and grain yield. It’s a known fact that various abiotic impacts during the growing season cause nonspecific changes in the metabolism of growth processes (2). The intensity and nature of plant response to a stressor depends on specific varietal (genetic) characteristics, as well as on the ontogeny phase.
Plant growth is a complex physiological process with high lability and sensitivity to fluctuations of internal and external factors (3, 4). Reproduction and propagation are basic functions of the organism (5). Vegetative cells and organs have significant genetic stability and reproducibility with apoptosis as a final act, while generative cells and tissues are involved in reproduction, which increases their genetic diversity during recombinations and mutations of determinants for different traits including resistance to stresses (6, 7).
The purpose of this study was ranking a wide range of spring wheat varieties for a number of morphological traits to form groups differing by resistance to stress (partial removal of photosynthesizing leaf surface).
Technique. The object of study – 45 varieties of soft spring wheat of different ecological and geographical origin: 10 - from Russia (including 5 cultivars of own selection), 4 – from Mexico, Germany, USA and The Netherlands, 3 - from Sweden, 2 - from the Ukraine, England and Poland, 1 variety - from India, Belarus, Australia, Finland, France, Yugoslavia, Czechoslovakia, Kazakhstan, Latvia and Canada. The studied genotypes were represented by six morphological types: Erythrospermum, Lutescens, Graecum, Albidum, Ferrugineum and Milturum.
Experiments were carried out in 2002-2005 in fields of of the Republic of Tatarstan (the forest-steppe zone, soil of a test area - black loam-textured chernozem).
Adaptability of the studied genotypes to adverse effects of stress was evaluated upon the reaction to removal of two upper leaves during the period of ear formation.
The indicators of stress response were determined in a phase of wax ripeness. Net photosynthesis was assessed from the total air-dry weight of aboveground parts. A stem was divided into the following parts: ear, flag leaf, ear shank (peduncle), 2nd leaf, 2nd internode, 3rd leaf, 3rd internode, which were dried to air-dry state, weighed and the obtained values were summed. Resistance of genotypes to stressful effects of a partial removal of leaf surface was evaluated in the phase of wax ripeness according to recommended algorithms (8-10).
Genotypic resistance of varieties to stressors was assessed using the own developed graphical method.
Statistical processing of data was performed in Microsoft Excel using the methods of variation statistics (11).
Results. Previously, a morphological comparison has revealed a high diversity of the analyzed cultivars for characteristics of plant growth, development of assimilating leaf surface, the dynamics of biomass accumulation in photosynthesizing organs during grain formation and ripening along with their unequal contribution to grain yield (12).
Grain formation and ripening are the processes related to creation of an offspring generation out of the photosynthesis in leaves and other green plant organs. Premature defoliation, abscission by leaf-eating insects, fungal diseases or experimental artificial deletion contribute to a stress and change in a quantitative relationship between producing plant organs and ones consuming assimilates, which, in turn, modifies the acceptor-donor ratio (ADR) of metabolism.
Biological productivity (plant weigh to harvest), or biological yield (weight of plants per area unit) reflect the net photosynthesis of a plant or agrocenosis during a growing season. As a plant growth, its metamers increase in size and weight with priority and dominance of acropetally younger metamers over the older (below) ones. In cereals, intercalary meristematic cells of ear shank develop the maximum attracting ability during a vegetative growth.
In this experiment, two upper leaves were removed in the period of ear formation as a stressor reducing leaf area surface and weight of photosynthetic organs, as well as the content of macro- and microelements providing ADR and resulting value of net photosynthesis.
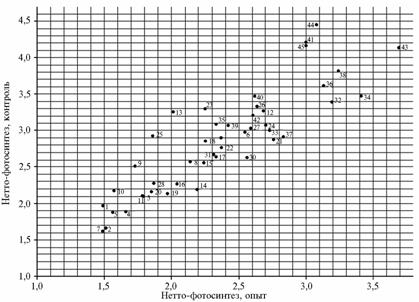
The authors propose a simple graphical method for estimating the genotypic resistance of varieties to stressors suitable for evaluation of impacts of any adverse factors. In these graphs, ordinates are the characteristics of net photosynthesis in control, abscissa shows the values obtained in the experiment (removal of leaves). In the given coordinates (X – experiment, Y – control) mean value of a trait is shown. Mean value over a test plot is known to be the genotypic value of a trait (9), so a cross point on the graph reflects genotypic characteristics for a particular trait manifested in each cultivar in experiment and control. The greater the difference between these values, the lower is genotypic stress tolerance in a particular cultivar.
A |
|
B |
|
|
|
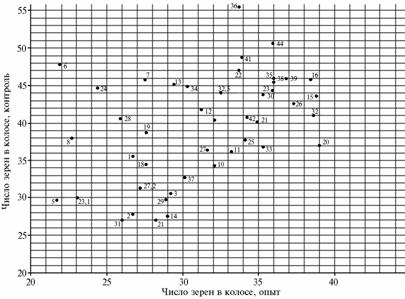
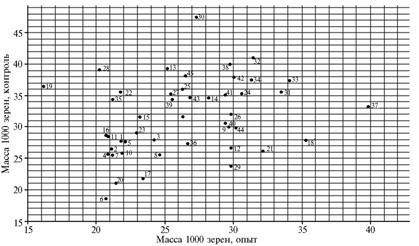
Graphical analysis of polygenic gene systems for net photosynthesis (g), number of grains per ear (pcs.) and acceptor ability of ear weevils (g) (respectively, A, B and C) after the deletion of two upper leaves in different varieties of soft wheat: 1 — W.S. 1809 (USA), 2 — Tobari 66 (Mexico), 3 — Sarrubra (Russia), 4 — Inia 66 (Mexico), 5 — HD 2307 (India), 6 — Red River 68 (USA), 7 — Pewek (Mexico), 8 — Conley (USA), 9 — Saratovskaya 60 (Russia), 10 — Kantegirskaya 89 (Russia), 11 — Fanori 71 (Mexico), 12 — Torres (Australia), 13 — AG-89-12 (Finland), 14 — Aniversario (USA), 15 — Balli (Germany), 16 — Dracon (Sweden), 17 — Sappo (Sweden), 18 — Kharkovskaya 18 (Ukraine), 19 — Highbury (England), 20 — Milton (Canada), 21 — Eritrospermum 609 (Russia), 22 — Hori-zont (Germany), 23 — Bastiаn (Netherlands), 24 — Rannyaya 93 (Ukraine), 25 — Melchior (Netherlands), 26 — Sirius (England), 27 — Troll (Sweden), 28 — Tyalve (Sweden), 29 — X 613 (Russia), 30 — Kleiber (Germany), 31 — HEC 182 (Poland), 32 — Heurtebise (France), 33 — Vidovica (Yugoslavia), 34 — Mephisto (Czechoslovakia), 35 — Ivolga (Russia), 36 — Ankra (Netherlands), 37 — Kazakhstanskaya 23 (Kazakhstan), 38 — Liva (Latvia), 39 — Sicco (Netherlands), 40 — Eta (Poland), 41 — Kerba (Russia), 42 — Debut (Russia), 43 — Eritrospermum 2 (Russia), 44 — Timer (Russia) (standard), 45 — Eritrospermum 3 (Russia). |
On the graph, a positive regression line (to the right and up) shows the cultivars most adaptive to the conditions of a corresponding field, climatic characteristics of a growing season and sowing thickness, while the points of the least adaptive varieties are located close to the point of origin (to the left and down). Genotypic differences in varieties’ response to the stressor enlarge the area of points along the horizontal axis.
It can be seen (Fig., A) that net photosynthesis of cv Saratovskaya 60 in control amounted to 2,5 g, in experiment – 1,7 g, whereas cv Kleiber developed equal values in both variants (2,7 g) with no reaction to the removal of two upper leaves. Cv Mephisto and Eritrospermum 2 manifested high values in control (resp., 3,5 and 4,1 g) and relatively high – in experiment (resp., 3,4 and 3,7 g), which indicates high stress tolerance of these varieties. On the contrary, Melchior, AG-89-12, Highbury, Conley, Bastian and other cultivars were found to be very sensitive.
It is known that after a triple fusion and syngamy, there occurs a reduce in intercalary meristematic activity in peduncle and vegetative parts of the inflorescence while the increase in rate of cell divisions in endosperm (13). In terms of physiology and genetics endosperm is distinct to a mother plant; this is a triploid body whose main function is active accumulation of assimilates (biological pump). The formation of chimaeric endospermic cells shifts ADR. Vegetative organs of a plant are formed and operate for 75-80% of a growing season, while grains (90% weight is endosperm) - within 3 weeks. Endospermic tissue is a transitional (between generations) acceptor-donor organ participating the development of a child generation and providing it with essential nutrients required by an embryo.
On a graphical model for the trait “number of grains per ear” (NGE) in conditions of the same stressor and group (Fig., B) there are shown many cultivars located above the horizontal level of 40 pcs. in control, bun none of them exceeds it in the experiment.
On a graph for the trait “weight of 1000 grains” (WG) under the same stressor (Fig., C), only three varieties were located above the horizontal line 40g in control – Kleiber, Heurtebise and Lebanon. In the experiment, the only cv Kazakhstanskaya 23 reached this level: WG = 40 g in control and 35 g in experiment. At the same time, in cv Kharkovskaya 18 WG amounted to 28 g (control) and 35 g (experiment). The varieties Red River 68, Milton, Sappo, Eritrospermum 609 and X613 also showed higher WG in the experiment compared with control. On the contrary, cv Tobari 66, Pewek, Kantegirskaya 89, Bastian, Balli, Hori-zont, Oriole, Highbury, and some others showed a smaller WG in the experiment, while no significant difference was observed in cv Conley, Eta, Inia 66 and HEC 182.
The phenomenon of increased weight of 1000 grains in plants after the deletion of two upper leaves compared to intact control can be explained by two factors: a reduced number of grains per ear (clearly expressed in cv Red River 68) and a weakened competition of two attracting centers - ear and upper leaves as the latter was experimentally removed (Kazakhstanskaya 23).
Analyzing the variation in stress tolerance among the studied varieties, it can be concluded that quantitative traits (net photosynthesis, number of grains per ear and weight of 1000 grains) were subject to unequal improvement during the selection. In the polygenic system of stress tolerance, net photosynthesis (Fig., A) is shown by the oval shaped cloud of points, number of grains per ear and grain size – a round shape.
Thus, a simple graphical method for evaluation of genotypic resistance of productivity traits under the stress (removal of two top leaves during the ear formation) was applied upon a set of 45 varieties of spring wheat, which has revealed a significant genotypic diversity useful for a further genetic improvement of wheat in respect to attracting ability of the ear.
REFERENCES
1. Zhuchenko A.A., Adaptivnoe rastenievodstvo (ecologo-geneticheskie osnovy) (Adaptive Plant Growing: Ecological and Genetic Basics), Kishinev, 1990.
2. Udovenko G.V., Resistance of Plants to Abiotic Stresses, in Fiziologicheskie osnovy selektsii (Physiological Basics of Selection), Udovenko G.V. and Shevelukha V.S., Eds., St. Petersburg, 1995, vol. 2, pp. 327-352.
3. Sytnik K.M., Fiziologo-biokhimicheskie osnovy rosta rastenii (Physiological and Biochemical Basics of Plant Growth), Kyiv, 1966.
4. Shevelukha V.S., Rost rastenii i ego regulyatsiya v ontogeneze (Plant Growth and Its Regulation in Ontogeny), Moscow, 1992.
5. Batygina T.B. and Vasilyeva V.E., Razmnozhenie rastenii (Propagation of Plants), St. Petersburg, 2002.
6. Samuilov V.D., Oleskin A.V. and Lyagunova E.M., Programmed Cell Death, Biokhimiya, 2000, vol. 65, no. 8, pp. 1029-1046.
7. Tarchevsky I.A., Signal’nye sistemy kletok rastenii (Signaling Systems of Plant Cells), Moscow, 2002.
8. Dragavtsev V.A., K probleme geneticheskogo analiza poligennykh priznakov rastenii (About the Problem of Genetic Analysis of Polygenic Traits in Plants), St. Petersburg, 2003.
9. Dragavtsev V.A., Otsenka zernovykh kul’tur po adaptivnosti i drugim poligennym sistemam (Evaluation of Adaptivity and Other Polygenic Traits in Cereal Crops), Dragavtsev V.A., Ed., St. Petersburg, 2002.
10. Dragavtsev V.A., Algoritmy ekologo-geneticheskoi inventarizatsii genofonda i konstruirovanie sortov sel’skokhozyaistvennykh rastenii po urozhainosti, ustoichivosti i kachestvu (Metodicheskie rekomendatsii, novye podkhody) (Algorithms for Ecological and Genetic Account of Gene Pools and Design of Cultivated Crops Varieties for Productivity, Resistance and Quality: Methodological Recommendations, New Approaches), St. Petersburg, 1994.
11. Dospekhov B.A., Metodika polevogo opyta (Technique of Field Experiment), Moscow, 1965.
12. Ionova N.E., Khokhlova L.P. and Valiullina R.N., Role of Individual Organs in Productive Process in Spring Wheat Plants of Different Ecologo-Geographical Origin, S.-kh. biol., 2009, no. 1, pp. 60-67.
13. Batygina T.B., Khlebnoe zerno (Bread Grain), Leningrad, 1987.
1Plant Breeding and Seed Growing Enterprise of Je.F. Ionov, Chistopol' 422980, The Republic of Tatarstan, Russia; |
Received February 25, 2011
|